Chapter: Organic Chemistry: Functional groups
Primary, secondary, tertiary and quaternary nomenclature
PRIMARY, SECONDARY, TERTIARY AND QUATERNARY
NOMENCLATURE
Key Notes
Definition
Carbon
centers, as well as some functional groups (alcohols, alkyl halides, amines and
amides), can be defined as primary (1°), secondary (2°),
tertiary (3°) or quaternary (4°).
Carbon centers
Carbon
centers can be identified as primary, secondary, tertiary, or quater-nary
depending on the number of bonds leading to other carbon atoms. A methyl group
contains a primary carbon center. A methylene group (CH2) contains a
secondary carbon center. The methine group (CH) contains a tertiary carbon
center while a carbon atom having four substituents is a quaternary center.
Amines and amides
Amines
and amides can be defined as being primary, secondary, tertiary, or quaternary
depending on the number of bonds leading from nitrogen to carbon.
Alcohols and alkyl halides
Alcohols
and alkyl halides are defined as primary, secondary, or tertiary depending on
the carbon to which the alcohol or halide is attached. The assignment depends
on the number of bonds from that carbon to other car-bon atoms. It is not
possible to get quaternary alcohols or quaternary alkyl halides.
Definition
The primary (1°), secondary (2°), tertiary (3°) and quaternary (4°) nomenclature is used in a variety of
situations: to define a carbon center, or to define functional groups such as
alcohols, halides, amines and amides. Identifying functional groups in this way
can be important since the properties and reactivities of these groups may vary
depending on whether they are primary, secondary,tertiary,or quaternary.
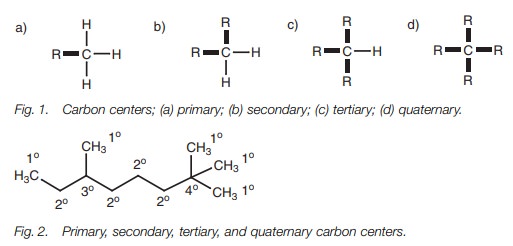
Carbon centers
One of the easiest ways of determining whether
a carbon center is 1°, 2°, 3° or 4° is to
count the number of bonds leading from that carbon center to another carbon
atom (Fig. 1). A methyl group (CH3) is a primary carbon center, a methylene group (CH2) is a
secondary carbon center, a methine
group (CH) is a tertiary carbon center, and a carbon center with four alkyl
substituents (C) is a quaternary carbon center (Fig. 2).
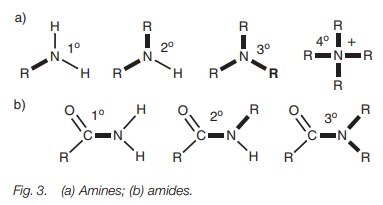
Amines and amides
Amines and amides can be defined as being
primary, secondary, tertiary, or qua- ternary depending on the number of bonds
from nitrogen to carbon (Fig. 3). Note that a quaternary amine is positively
charged and is therefore called a quaternary ammonium ion. Note also that it is
not possible to get a quaternary amide.
Alcohols and alkyl halides
Alcohols and alkyl halides can also be defined as being primary, secondary, or ter- tiary (Fig. 4). However, the definition depends on the carbon to which the alcohol or halide is attached and it ignores the bond to the functional group. Thus, qua- ternary alcohols or alkyl halides are not possible.
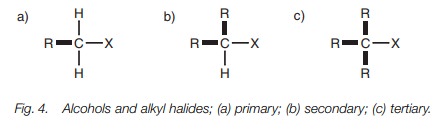
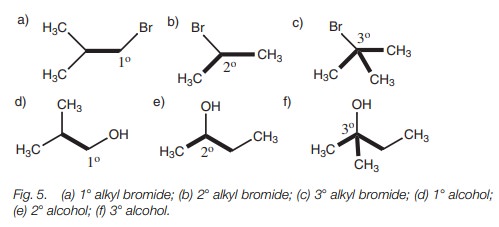
The following examples (Fig. 5) illustrate different types of alcohols and alkyl halides.
Related Topics