Chapter: Organic Chemistry: Functional groups
Intermolecular bonding
INTER MOLECULAR BONDING
Key Notes
Definition
Intermolecular
bonding takes place between different molecules. This can take the form of
ionic bonding, hydrogen bonding, dipole–dipole inter-actions and van der Waals
interactions. The type of bonding involved depends on the functional groups
present.
Ionic bonding
Ionic
bonds are possible between ionized functional groups such as car-boxylic acids
and amines.
Hydrogen bonding
Intermolecular
hydrogen bonding is possible for alcohols, carboxylic acids, amides, amines,
and phenols. These functional groups contain a hydrogen atom bonded to nitrogen
or oxygen. Hydrogen bonding involves the inter-action of the partially positive
hydrogen on one molecule and the partially negative heteroatom on another
molecule. Hydrogen bonding is also possi-ble with elements other than nitrogen
or oxygen.
Dipole–dipole interactions
Dipole–dipole
interactions are possible between molecules having polariz-able bonds, in
particular the carbonyl group (C=O).
Such bonds have a dipole moment and molecules can align themselves such that
their dipole moments are parallel and in opposite directions. Ketones and aldehydes
are capable of interacting through dipole–dipole interactions.
van der Waals interactions
van der
Waals interactions are weak intermolecular bonds between regions of different
molecules bearing transient positive and negative charges. These transient
charges are caused by the random fluctuation of electrons. Alkanes, alkenes,
alkynes and aromatic rings interact through van der Waals interactions.
Definition
Intermolecular bonding is the bonding interaction which takes place
between different molecules. This
can take the
form of ionic bonding,
hydrogenbonding, dipole–dipole interactions or van der Waals interactions. These bonding forces are weaker than
the covalent bonds,but they have an important influence on the physical and
biological properties of a compound.
Ionic bonding
Ionic bonding takes place between molecules
having opposite charges and involves an electrostatic
interaction between the two opposite charges. The functional groups which most
easily ionize are amines and carboxylic acids (Fig. 1).
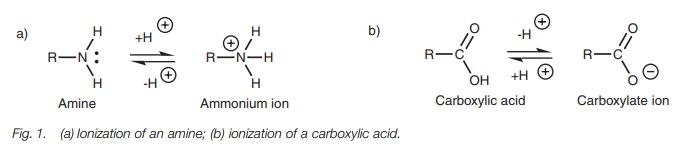
Ionic bonding is possible between a molecule
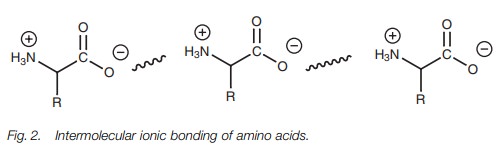
containing an ammonium ion and a molecule containing a carboxylate ion. Some
important naturally occurring mol-ecules contain both groups – the amino acids.
Both these functional groups are ionized to form a structure known as a zwitterion (a neutral molecule bearing
both a positive and a negative charge) and intermolecular ionic bonding can
take place (Fig. 2).
Hydrogen bonding
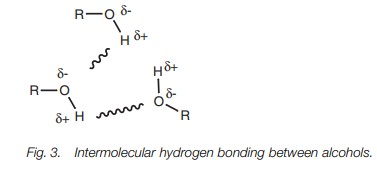
Hydrogen bonding can take place when molecules
have a hydrogen atom attached to a heteroatom such as nitrogen or oxygen. The
common functional groups which can participate in hydrogen bonding are
alcohols, phenols, carboxylic acids, amides, and amines. Hydrogen bonding is
possible due to the polar nature of the N–H or O–H bond. Nitrogen and oxygen
are more electronegative than hydrogen. As a result, the heteroatom gains a
slightly negative charge and the hydrogen gains a slightly positive charge.
Hydrogen bonding involves the partially charged hydrogen of one molecule (the H bonddonor) interacting with the
partially charged heteroatom of another molecule (the H bond acceptor) (Fig. 3).
Dipole–dipole interactions
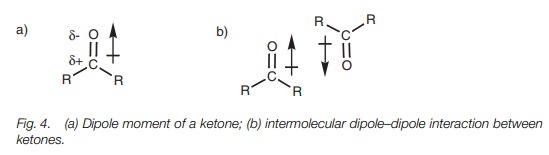
Dipole–dipole interactions are possible between
polarized bonds other than N–H or O–H bonds. The most likely functional groups
which can interact in this way are those containing a carbonyl group (C=O). The electrons in the carbonyl bond are
polarized towards the more electronegative oxygen such that the oxygen gains a
slight negative charge and the carbon gains a slight positive charge. This
results in a dipole moment which can be represented by the arrow shown in Fig. 4. The arrow points to the negative
end of the dipole moment. Molecules containing dipole moments can align
themselves with each other such that the dipole moments are pointing in
opposite directions (Fig. 4b).
van der Waals interactions
van der Waals interactions are the weakest of
the intermolecular bonding forces and involve the transient existence of
partial charges in a molecule. Electrons are continually moving in an
unpredictable fashion around any molecule. At any moment of time, there is a
slight excess of electrons in one part of the molecule and a slight deficit in
another part. Although the charges are very weak and fluctuate around the
molecule, they are sufficiently strong to allow a weak interaction between
molecules, where regions of opposite charge in different molecules attract each
other.
Alkane molecules can interact in this way and
the strength of the interaction increases with the size of the alkane molecule.
van der Waals interactions are also important for alkenes, alkynes and aromatic
rings. The types of molecules involved in this form of intermolecular bonding
are ‘fatty’ molecules which do not dissolve easily in water and such molecules
are termed hydrophobic
(water-hating). Hydrophobic molecules can dissolve in nonpolar, hydrophobic
solvents due to van der Waals interactions and so this form of intermolecular
bonding is sometimes referred to as a hydrophobic interaction.
Related Topics