Chapter: Organic Chemistry: Functional groups
Nomenclature of compounds with functional groups
NOMENCLATURE OF COMPOUNDS WITH FUNCTIONAL
GROUPS
Key Notes
General rules
The main
chain (or parent chain) must include the functional group. The presence of
functional groups is indicated by adding the relevant suffix for that
functional group. The position of the functional group must be defined and
other substituents are identified as described for alkanes.
Alkenes and alkynes
Alkenes and
alkynes are defined
by adding the
suffixes -ene and
-yne respectively. The stereochemistry of alkenes may need to be
defined.
Aromatics
The
simplest aromatic ring is benzene. Other important aromatic molecules include
toluene, phenol, aniline, benzoic acid, and benzaldehyde. Any of these names
can be used as parent names if other substituents are present. The posi- tion
of substituents is determined by numbering round the ring, or in the case of
disubstituted aromatic rings, the ortho, meta, para nomenclature.
Alcohols
Alcohols
(or alkanols) are given the suffix -anol.
Ethers and alkyl halides
Ethers
and alkyl halides are not identified with suffixes. Instead, these func-tional
groups are considered to be substituents of the main alkane chain. The halogen
of an alkyl halide is a halo substituent, while the ether is an alkoxy
substituent.
Aldehydes and ketones
Aldehydes
(or alkanals) are identified by the suffix -anal. Ketones (or alka-nones) are
identified by the suffix -anone. Aldehydes must always be at position 1 of the
main chain and do not need to be numbered.
Carboxylic acids and acid chlorides
Carboxylic
acids and acid chlorides are identified by adding the suffix -anoic acid and
-anoyl chloride respectively. Both these functional groups are always at the
end of the main chain and do not need to be numbered.
Esters
Esters
are named from the parent carboxylic acid and alcohol. The alkanoic acid is
renamed alkanoate and the alkanol is treated as an alkyl substituent. The
combined name is alkyl alkanoate. There must be a space between both parts of
the name.
Amides
Amides
are termed as alkanamides based on the parent carboxylic acid. If the amide
nitrogen has alkyl groups, then these are considered as alkyl sub-stituents. The
symbol N is used to show that the
substituents are on the nitrogen and not some other part of the alkanamide
skeleton.
Amines
Simple
amines can be named by placing the suffix -ylamine after the root name. Other
amines are named by considering the amino group as a sub-stituent of the main
chain in the same way as alkyl halides and ethers.
Thiols and thioethers
Thiols
are named by adding the suffix thiol to the name of the main alkane chain.
Thioethers are named in the same way as ethers where the major alkyl
substituent is considered to be the main chain with an alkylthio sub-stituent.
Simple thioethers can be identified as dialkylsulfides.
General rules
Many of the nomenclature rules for alkanes hold
true for molecules containing a functional group, but extra rules are needed in
order to define the type of functional group present and its position within
the molecule. The main rules are as follows:
i.The main (or parent) chain must include the
functional group, and so may not necessarily be the longest chain (Fig. 1);
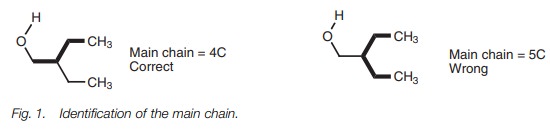
ii.The presence of some functional groups is
indicated by replacing -ane for the parent alkane chain with the following
suffixes:
iii.Numbering must start from the end of the
main chain nearest the functional group. Therefore, the numbering should place
the alcohol (Fig. 2) at position 1
and not position 4.

iv.The position of the functional group must be
defined in the name. Therefore, the alcohol (Fig. 2) is a 1-butanol.
v.Other substituents are named and ordered in
the same way as for alkanes. The alcohol (Fig.
2) has an ethyl group at position 2 and so the full name for the structure
is 2-ethyl-1-butanol.

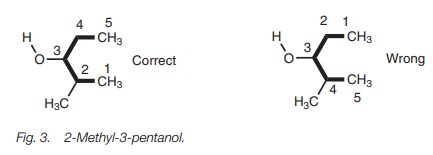
There are other rules designed for specific situations. For example, if the func-tional group is an equal distance from either end of the main chain, the number-ing starts from the end of the chain nearest to any substituents. For example, the alcohol (Fig. 3) is 2-methyl-3-pentanol and not 4-methyl-3-pentanol.
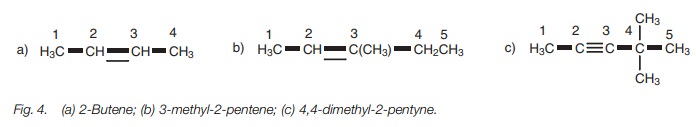
Alkenes and alkynes
Alkenes and alkynes have the suffixes -ene and
-yne respectively (Fig. 4). With some
alkenes it is necessary to define the stereochemistry of the double bond.
Aromatics
The best known aromatic structure is benzene.
If an alkane chain is linked to a benzene molecule, then the alkane chain is
usually considered to be an alkyl substituent of the benzene ring. However, if
the alkane chain contains more than six carbons, then the benzene molecule is
considered to be a phenyl substituent of the alkane chain (Fig. 5).
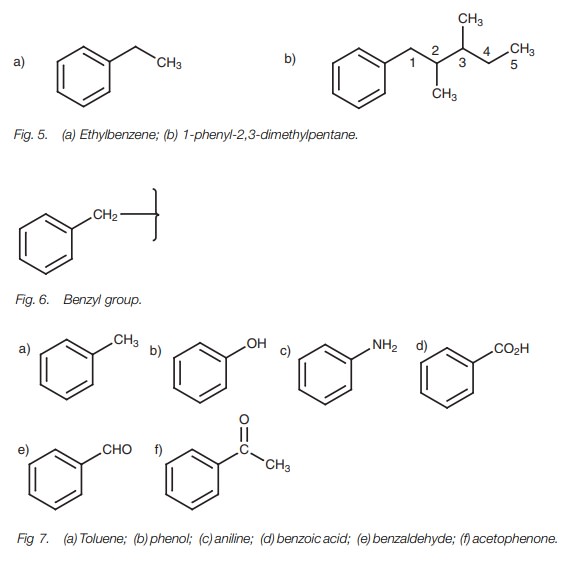
Note that a benzyl group consists of an aromatic ring and a methylene group
Benzene is not the only parent name which can be used for aromatic com- pounds (Fig. 7).
With disubstituted aromatic rings, the position
of substituents must be defined by numbering around the ring such that the
substituents are positioned at the lowest numbers possible, for example, the
structure (Fig. 8) is
1,3-dichlorobenzene and not 1,5-dichlorobenzene.

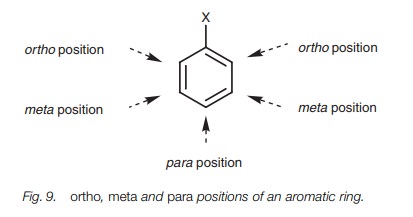
Alternatively, the terms ortho, meta, and para can be used. These terms define the
relative position of one substituent to another (Fig. 9). Thus, 1,3-dichlorobenzene can also be called meta-dichlorobenzene. This can be
shortened to m-dichlorobenzene. The
examples in Fig. 10 illustrate how
different parent names may be used. Notice that the substituent which defines
the parent name is defined as position 1. For example, if the parent name is
toluene, the methyl group must be at position 1.
When more than two substituents are present on the aromatic ring, the ortho, meta, para nomenclature is no longer valid and numbering has to be used (Fig. 11).
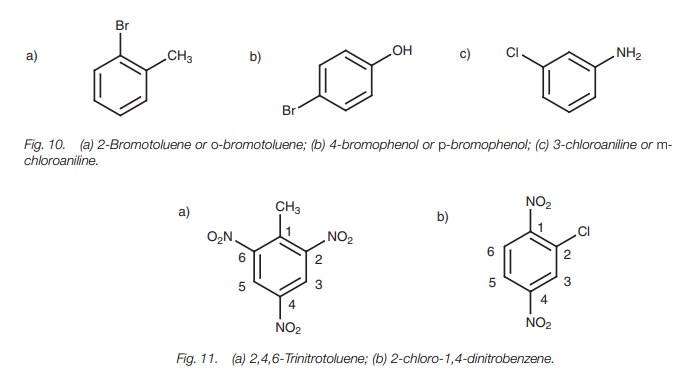
Once again, the relevant substituent has to be placed at position 1 if the parent name is toluene, aniline, et cetera. If the parent name is
benzene, the num-bering is chosen such that the lowest possible numbers are
used. In the example shown, any other numbering would result in the
substituents having higher numbers (Fig.
12).

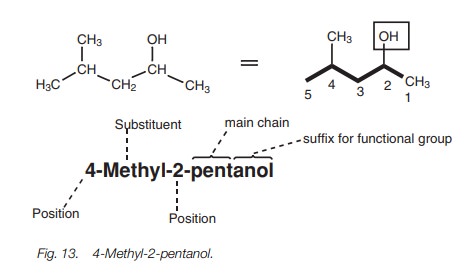
Alcohols
Alcohols or alkanols are identified by using the suffix -anol. The general
rules described earlier can be used to name alcohols (Fig. 13).
Ethers and alkyl halides
The nomenclature for these compounds is
slightly different from previous exam- ples in that the functional group is
considered to be a substituent of the main alkane chain. The functional group
is numbered and named as a substituent (Fig.
14).
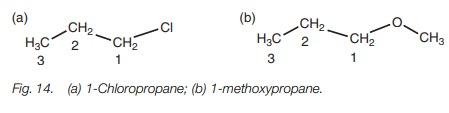
Note that ethers have two alkyl groups on
either side of the oxygen. The larger alkyl group is the parent alkane. The
smaller alkyl group along with the oxygen is the substituent and is known as an
alkoxy group.
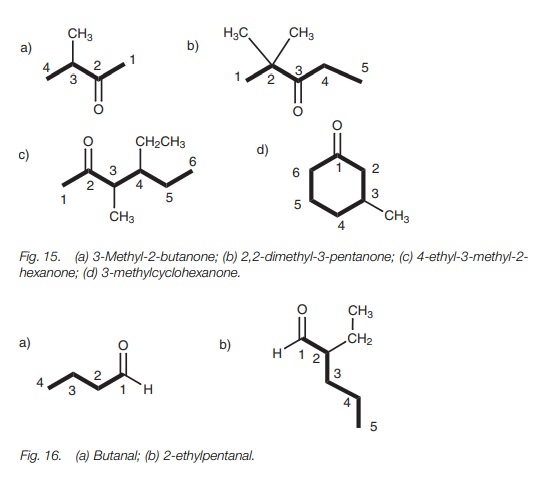
Aldehydes and ketones
The suffix for an aldehyde (or alkanal) is
-anal, while the suffix for a ketone (or alkanone) is -anone. The main chain
must include the functional group and the numbering is such that the functional
group is at the lowest number possible. If the functional group is in the
center of the main chain, the numbering is chosen to ensure that other
substituents have the lowest numbers possible (e.g. 2,2-dimethyl-3-pentanone
and not 4,4-dimethyl-3-pentanone; Fig. 15).
3-Methyl-2-butanone can in fact be simplified to 3-methylbutanone. There is
only one possible place for the ketone functional group in this molecule. If
the carbonyl C=O group was at the end of the chain, it would be an aldehyde and
not a ketone. Numbering is also not necessary in locating an aldehyde group
since it can only be at the end of a chain (Fig.
16).
Carboxylic acids and acid chlorides
Carboxylic acids and acid chlorides are
identified by adding the suffix -anoic acid and -anoyl chloride respectively. Both these functional groups are
always at the end of the main chain and do not need to be numbered (Fig. 17).

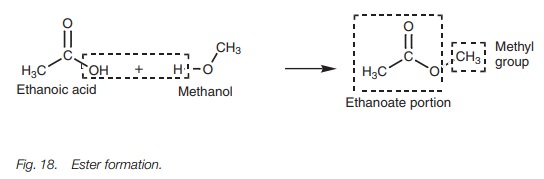
Esters
To name an ester, the following procedure is
carried out:
1.
identify the carboxylic acid (alkanoic acid) from which it was
derived;
2.
change the name to an alkanoate rather than an alkanoic acid;
3.
identify the alcohol from which the ester was derived and consider
this as an alkyl substituent;
4.
the name becomes an alkyl alkanoate.
For example, the ester (Fig. 18) is derived from ethanoic acid and methanol. The ester
would be an alkyl ethanoate since it is derived from ethanoic acid. The alkyl
group comes from methanol and is a methyl group. Therefore, the full name is
methyl ethanoate. (Note that there is a space between both parts of the name.)
Amides
Amides are also derivatives of carboxylic
acids. This time the carboxylic acid is linked with ammonia or an amine. As
with esters, the parent carboxylic acid is identified. This is then termed an alkanamide and includes the nitrogen
atom. For example, linking ethanoic acid with ammonia gives ethanamide (Fig. 19).
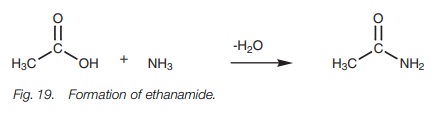
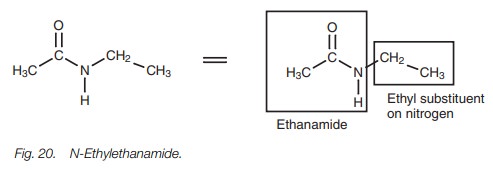
If the carboxylic acid is linked with an amine,
then the amide will have alkyl groups on the nitrogen. These are considered as
alkyl substituents and come at the beginning of the name. The symbol N is used to show that the substituents
are on the nitrogen and not some other part of the alkanamide skeleton. For
example, the structure in Fig. 20 is
called N-ethylethanamide.
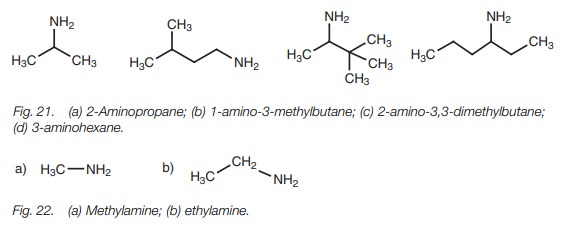
Amines
The nomenclature for amines is similar to alkyl
halides and ethers in that the main part (or root) of the name is an alkane and
the amino group is considered to be a substituent (Fig. 21). Simple amines are sometimes named by placing the suffix
-ylamine after the main part of the name (Fig.
22).
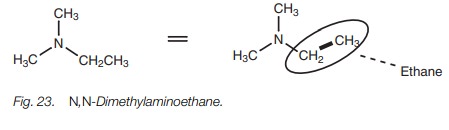
Amines having more than one alkyl group
attached are named by identifying the longest carbon chain attached to the
nitrogen. In the example (Fig. 23),
that is an ethane chain and so this molecule is an aminoethane (N,N-dimethylaminoethane).
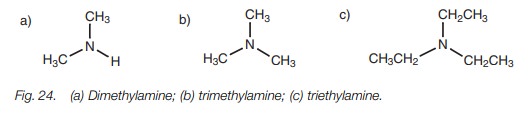
Some simple secondary and tertiary amines have
common names (Fig. 24).
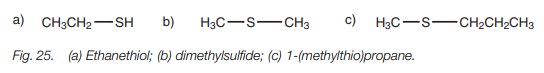
Thiols and thioethers
Thiols are named by adding the suffix thiol to the name of the parent alkane
(Fig. 25a). Thioethers are named in
the same way as ethers using the prefixalkylthio,
for example, 1-(methylthio)propane (Fig.
25c). Simple thioethers can be named by identifying the thioether as a sulfide and prefixing this term with
the alkyl sub- stituents, for example, dimethyl sulfide (Fig. 25b).
Related Topics