Chapter: Organic Chemistry: Functional groups
Functional groups: Properties and reactions
PROPERTIES AND REACTIONS
Key Notes
Properties
The
presence of functional groups affect such properties as melting points, boiling
points, polarity, dipole moments, and solubility. Molecules with strongly polar
functional groups tend to have higher melting points and boiling points than
molecules with nonpolar functional groups, and prefer to dissolve in polar solvents
rather than nonpolar solvents.
Reactions
The
sorts of reactions which compounds undergo are determined by the sorts of
functional groups which are present. Functional groups undergo characteristic
reactions, but the rates of these reactions are affected by stereoelectronic
factors and conjugation.
Properties
The chemical and physical properties of an
organic compound are determined by the sort of intermolecular bonding forces
present, which in turn depends on the functional group present. A molecule such
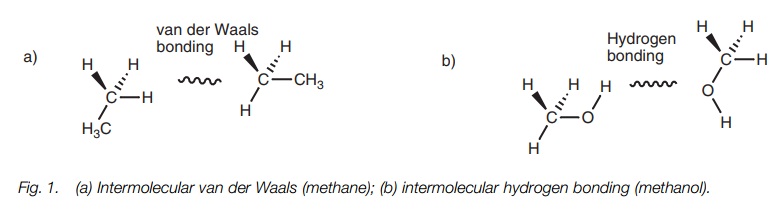
as ethane has a low boiling point and is a gas at room temperature because its
molecules are bound together by weak van der Waals forces (Fig. 1a). In contrast, methanol is a liquid at room temperature
since hydrogen bonding is possible between the alcoholic functional groups (Fig. 1b).
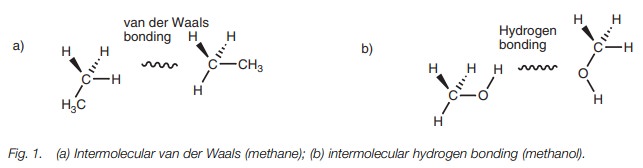
The polarity of molecules depends on which
functional groups are present. A molecule will be polar and have a dipole
moment if it contains polar functional groups such as an alcohol, amine, or
ketone. Polarity also determines solubility in different solvents. Polar
molecules prefer to dissolve in polar solvents such as water or alcohols,
whereas nonpolar molecules prefer to dissolve in nonpolar sol-vents such as
ether and chloroform. Polar molecules which can dissolve in water are termed hydrophilic (water-loving) while
nonpolar molecules are termed hydrophobic
(water-hating).
In most cases, the presence of a polar
functional group will determine the phys-ical properties of the molecule.
However, this is not always true. If a molecule has a polar group such as a
carboxylic acid, but has a long hydrophobic alkane chain, then the molecule
will tend to be hydrophobic.
Reactions
The vast majority of organic reactions take
place at functional groups and are characteristic of that functional group.
However, the reactivity of the functional group is affected by stereoelectronic effects. For example,
a functional group may be surrounded by bulky groups which hinder the approach
of a reagent and slow down the rate of reaction. This is referred to as steric shielding. Electronic effects can also influence the rate of a reaction.
Neighboring groups can influence the reactivity of a functional group if they
are electron-withdrawing or electron-donating and influence the electronic
density within the functional group. Conjugation and aromaticity also has an
important effect on the reactivity of functional groups. For example, an
aromatic ketone reacts at a different rate from an aliphatic ketone. The
aromatic ring is in conjugation with the carbonyl group and this increases the
stability of the overall system, making it less reactive
Related Topics