Chapter: Organic Chemistry: Stereochemistry
Constitutional isomers
CONSTITUTIONAL ISOMERS
Key Notes
Introduction
Isomers
are compounds which have the same molecular formula, but differ in the way the
atoms are arranged. There are three types of isomers – constitutional, configurational,
and conformational.
Definition
Constitutional
isomers are compounds which have the same molecular for-mula but have the atoms
joined together in a different way. Constitutional isomers have different
physical and chemical properties.\
Alkanes
Alkanes
of a particular molecular formula can have various constitutional isomers. The
larger the alkane, the more isomers which are possible.
Introduction
Isomers are compounds which have the same
molecular formula (i.e. they have the same atoms), but differ in the way these
atoms are arranged. There are three types
of isomers –
constitutional isomers, configurational isomers,
and conformational isomers. Constitutional isomers are isomers where the
atoms arelinked together in a different skeletal framework and are different
compounds.Configurational isomers are structures having the same atoms and
bonds, butwhichhave different geometrical shapes which cannot be interconverted
withoutbreaking covalent bonds.
Configurational isomers can be separated
and are different compounds
with different properties.
Conformational isomers are different shapes of the same molecule and
cannot be separated.
Definition
Constitutional isomers are compounds which have
the same molecular formula but have the atoms joined together in a different
way. In other words, they have different carbon skeletons. Constitutional
isomers have different physical and chemical properties.
Alkanes
Alkanes of a particular molecular formula can
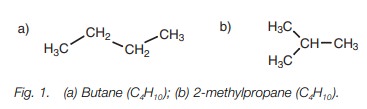
exist as different constitutional isomers. For example, the alkane having the
molecular formula C4H10 can exist as two constitutional isomers – the straight
chain alkane (butane) or the branched alkane (2-methylpropane; Fig. 1). These
are different compounds with different physical and chemical properties.
Related Topics