Chapter: Organic Chemistry: Stereochemistry
Configurational isomers - optical isomers
CONFIGURATIONAL ISOMERS ŌĆō OPTICAL ISOMERS
Key Notes
Definition
Optical
isomers are configurational isomers which have the ability to rotate plane-polarized
light clockwise or counterclockwise. They have identical chemical and physical
properties (apart from their effects on plane-polarized light), but can have
different biological properties.
Asymmetric molecules
Asymmetric
molecules are molecules which lack symmetry. Such molecules can also be termed
as chiral molecules and as such can exist as two non-superimposable mirror
images. These mirror images are optical isomers and are a form of
configurational isomerism. Complete asymmetry is not required for a molecule to
be chiral and some chiral molecules can have a single axis of symmetry.
Asymmetric carbon centers
Asymmetric
carbon centers are carbon atoms having four different sub-stituents. Molecules
having asymmetric centers will usually be chiral. How-ever, there are special
cases where molecules can have asymmetric centers and be achiral, or where
molecules are chiral but have no asymmetric cen-ters. Nonsuperimposable mirror
images of a molecule are called enan-tiomers. An equal mixture of two enantiomers
is called a racemate and does not rotate plane polarized light. Asymmetric
carbon centers are only possi-ble on sp3
carbons.
Fischer diagrams
Fischer
diagrams are used to represent chiral molecules. The vertical bonds in a
Fischer diagram represent bonds pointing into the page while the horizontal
bonds represent bonds coming out of the page.
R and S nomenclature
The R and S nomenclature is used to determine the absolute configuration at
asymmetric centers. The groups attached to the asymmetric center are given
priorities based on the atomic weights of the atoms directly attached to the
center. The group of lowest priority is placed behind the page and an arc is
drawn connecting the top three priority groups. If the arc is clockwise, the
assignment is R. If the arc is
anticlockwise, the assignment is S.
(+) and (ŌĆō)
The
symbols (+) and (-) are used to show which direction an enantiomer rotates
plane-polarized light. The direction of rotation can only be deter-mined by
experimentation.
Optical purity
Optical
purity is quoted in terms of enantiomeric excess. This indicates the excess of
pure enantiomer over racemate.
Allenes and spiro compounds
Some
substituted allenes and spiro compounds are chiral molecules despite the lack
of an asymmetric center. Molecules are chiral if they are asymmet-ric overall
or contain only one axis of symmetry.
meso structures
A meso structure has two identical
asymmetric centers which effectively cancel each other out. Such a molecule has
a plane of symmetry and cannot be chiral. The mirror images are superimposable
and optical isomers are not possible.
Diastereomers
Molecules
containing more than one asymmetric center can have several stereoisomers, that
is, different configurational isomers. For each asymmet-ric center present (n) in a molecule, there are 2n possible stereoisomers.
Every stereoisomer has a mirror image and so there are 2n-1 sets of enan-tiomers. Each set of
enantiomers is called a diastereomer. Diastereomers are different molecules
having different chemical and physical properties.
Definition
Optical isomerism is another example of configurational isomerism and is so named because
of the ability of optical isomers to rotate plane-polarized light clockwise or
counterclockwise. The existence of optical isomers has very important consequences
for life, since
optical isomers often
have significant differences in their
biological activity. Apart from their biological activity and their
effects on plane-polarized light,
optical isomers have
identical chemical and physical properties.
Asymmetric molecules
A molecule such as chloroform (CHCl3)
is tetrahedral and there is only one way of fitting the atoms together. This is
not the case for a molecule such as lactic acid.
There are two ways of constructing a model of
lactic acid, such that the two struc- tures
obtained are nonsuperimposable and
cannot be interconverted without breaking covalent bonds. As such,
they represent two different molecules which are configurational isomers. The
difference between the two possible molecules lies in the way the substituents
are attached to the central carbon. This can be rep- resented by the following
drawings (Fig. 1) where the bond to
the hydroxyl group comes out of the page in one isomer but goes into the page
in the other isomer.
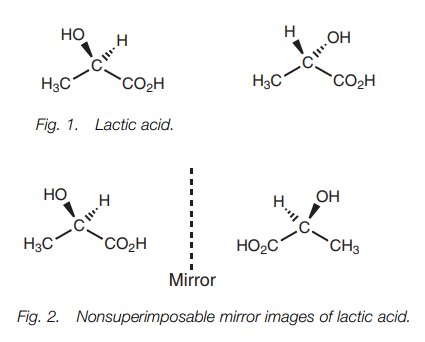
The two isomers of lactic acid are mirror images (Fig. 2). A molecule which exists as two nonsuperimposable mirror images has optical activity if only one of the mirror images is present.
Lactic acid exists as two nonsuperimposable
mirror images because it is asym- metric
ŌĆō in other words it lacks symmetry. Asymmetric molecules can also betermed
as chiral, and the ability of
molecules to exist as two optical isomers iscalled chirality. In fact, a molecule does not have to be totally
asymmetric to bechiral. Molecules containing a single axis of symmetry can also
be chiral.
Asymmetric carbon centers
A simple method of identifying most chiral
molecules involves identifying what are known as asymmetric carbon centers. This works for most chiral molecules ,but
it is important to realize that it is not foolproof and that there are several
cases where it will not work. For example, some chiral molecules have no
asymmetric carbon centers, and some molecules having more than one asymmetric
carbon center are not chiral.
Usually, a compound will have optical isomers if there are four different sub- stituents attached to a central carbon (Fig. 3). In such cases, the mirror images are nonsuperimposable and the structure will exist as two configurational isomers called enantiomers. The carbon center which contains these four different sub- stituents is known as a stereogenic or an asymmetric center. A solution of each enantiomer or optical isomer is capable of rotating plane-polarized light. One enantiomer will rotate plane-polarized light clockwise while the other (the mirrorimage) will rotate it counterclockwise by the same amount. A mixture of the two isomers (a racemate) will not rotate plane-polarized light at all. In all other respects, the two isomers are identical in physical and chemical properties and aretherefore indistinguishable. The asymmetric centers in the molecules shown(Fig. 4) have been identified with an asterisk. The structure lacking the asymmetriccenter is symmetric or achiral and does not have optical isomers. A structure canalso have more than one asymmetric center.
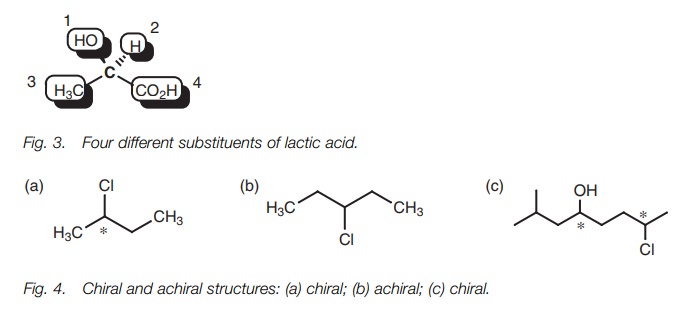
Asymmetric centers are only possible on sp3 carbons. Ansp2 center is planar and
cannot be asymmetric. Similarly, ansp
center cannot be asymmetric.
Fischer diagrams
A chiral molecule can be represented by a
Fischer diagram (Fig. 5). The molecule is
drawn such that
the carbon chain
is vertical with
the functional group positioned at the top. The vertical CŌĆōC
bonds from the asymmetric center point into the page while the horizontal bonds
from the asymmetric center come out of the page. This is usually drawn without
specifying the wedged and hatched bonds.
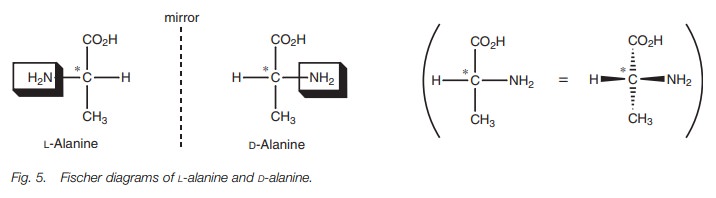
The Fischer diagrams of alanine allow the
structures to be defined as L- or D- from the position of the amino group. If
the amino group is to the left, then it is the L- enantiomer. If it is
positioned to the right, it is the D-enantiomer. This is an old fashioned
nomenclature which is only used for amino acids and sugars. The L- and D-
nomenclature depends on the absolute configuration at the asymmetric center and
not the direction in which the enantiomer rotates plane-polarized light. It is
not possible to predict which way a molecule will rotate plane-polarized light
and this can only be found out by experimentation.
R and S nomenclature
The structure of an enantiomer can be specified
by the R and S nomenclature, determined by the CahnŌĆōIngoldŌĆōPrelog rules. The
example (Fig. 6) shows how the nomenclature is worked out. First of all, the
atoms directly attached to the asym- metric center and their atomic numbers are
identified. Next, you give the attached atoms a priority based on their atomic
numbers. In this example, there are two car- bon atoms with the same atomic
numbers and so they cannot be given a priority. When this happens, the next
stage is to move to the next atom of highest atomic number (Fig. 7a). This
means moving to an oxygenfor one of the carbons and to a hydrogen for the
other.
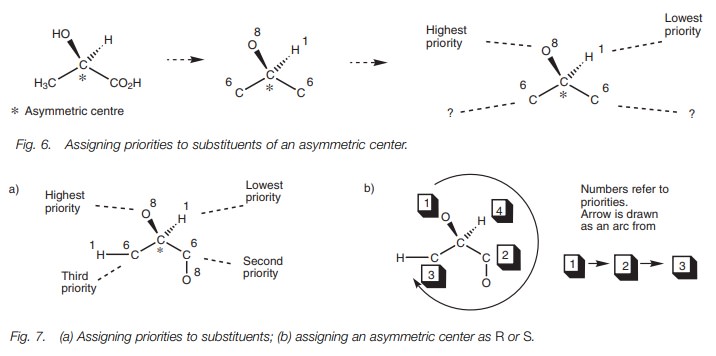
The oxygen has the higher priority and so this substituent takes priority over the other. Once the priorities have been settled, the structure is redrawn such that the group of lowest priority is positioned ŌĆśbehind the pageŌĆÖ.
In this example (Fig. 7b), the group of lowest priority (the hydrogen) is
already positioned behind the page (note the hatched bond indicating the bond
going away from you). An arc is now drawn connecting the remaining groups,
starting from the group of highest priority and finishing at the group of third
priority. If the arc is drawn clockwise, the assignment is R (rectus). If the arc is drawn counterclockwise, the assignment is
S (sinister). In this example the
arrow is drawn clockwise. Therefore, the molecule is (R)-lactic acid.
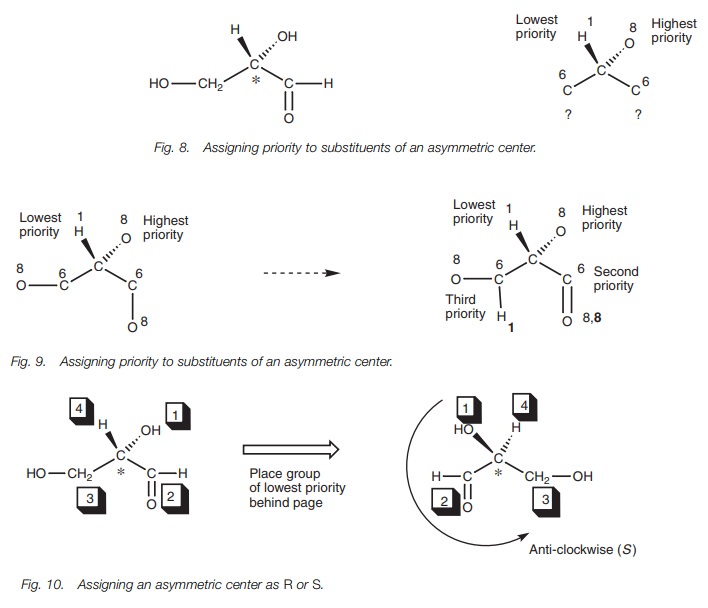
A second example (Fig. 8) illustrates another rule involving substituents with double bonds. The asymmetric center is marked with an asterisk. The atoms directly attached to the asymmetric center are shown on the right with their atomic numbers. At this stage, it is possible to define the group of highest priority (the oxygen) and the group of lowest priority (the hydrogen). There are two iden-tical carbons attached to the asymmetric center so we have to move to the next stage and identify the atom with the highest atomic number joined to each of the identical carbons (Fig. 9). This still does not distinguish between the CHO and CH2OH groups since both carbon atoms have an oxygen atom attached. The next stage is to look at the second most important atom attached to the two carbon atoms. However, if there is a double bond present, you are allowed to ŌĆśvisitŌĆÖ the same atom twice. The next most important atom in the CH2OH group is the hydrogen. In the CHO group, the oxygen can be ŌĆśrevisitedŌĆÖ since there is a double bond. Therefore, this group takes priority over the CH2OH group. The priorities have been determined, and so the group of lowest priority is placed behind the page and the three most important groups are connected to see if they are clock-wise or counterclockwise (Fig. 10).
(+) and (ŌĆō)
The assignment of an asymmetric center as R or S
has nothing to do with whichever direction the molecule rotates plane-polarized
light. Optical rotation can only be determined experimentally. By convention,
molecules which rotate plane-polarized light clockwise are defined as (+) or d. Molecules which rotate
plane-polarized light counterclockwise are defined as (-) or l. The R enantiomer of lactic acid is found to rotate plane-polarized
lightcounterclockwise and so this molecule is defined as (R)-( )-lactic acid.
Optical purity
The optical purity of a compound is a measure
of its enantiomeric purity and is given in terms of its enantiomeric excess (ee). A pure enantiomer would have an optical
purity and enantiomeric excess of 100%. A fully racemized compound would have
an optical purity of 0%. If the enantiomeric excess is 90%, it signifies that
90% of the sample is pure enantiomer and the remaining 10% is a racemate
containing equal amounts of each enantiomer (i.e. 5% + 5%). Therefore the ratio
of enantiomers in a sample having 90% optical purity is 95:5.
Allenes and spirocompounds
Not all chiral molecules have asymmetric
centers. For example, some substituted
allenes and spiro structures have no asymmetric center but are still
chiral (Fig. 11). The substituents at
either end of the allene are in different planes, and the rings in the spiro
structure are at right angles to each other. The mirror images of the allene
and the spiro structures are nonsuperimposable and are enantiomers.
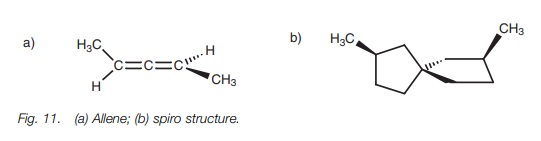
A better rule for determining whether a molecule
is chiral or not is to study the symmetry of the molecule. A molecule will be
chiral if it is asymmetric (i.e. has no elements of symmetry) or if it has no more than one axis of
symmetry.
mesostructures
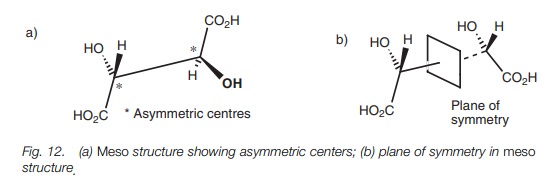
The molecule in Fig. 12a has two identical asymmetric centers but is not chiral. The mirror images of this structure are superimposable and so the compound cannot be chiral. This is because the molecule contains a plane of symmetry as demonstrated in Fig. 12b where the molecule has been rotated around the central CŌĆōC bond. A structure such as this is called a meso structure.
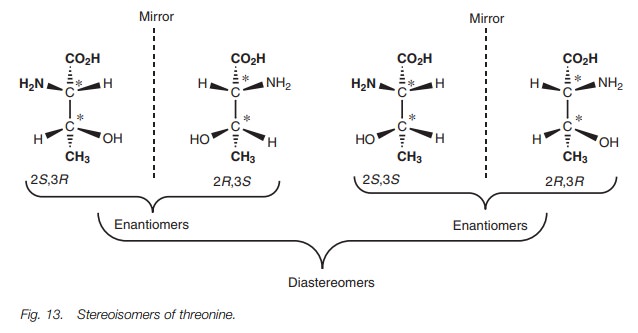
Diastereomers
Whenever a molecule has two or more asymmetric
centers, there are several possible structures which are possible and we need
to use terms such as stereoisomers, diastereomers and enantiomers in order
to discuss them. To illustrate the relativemeaning of these terms, we shall
look at the possible structures of the amino acid threonine (Fig. 13). This molecule has two
asymmetric centers. As a result, four different structures are possible arising
from the two different possible configurations at each center. These are
demonstrated with the asymmetric centers at positions 2 and 3 defined as R or S.
The four different structures are referred to as stereoisomers. The (2S,3R)
stereoisomer is a nonsuperimposable mirror image of the (2R,3S) stereoisomer and so
these structures are enantiomers having the same chemical and physical
properties. The (2S,3S) stereoisomer is the nonsuperimposable
mirror image of the (2R,3R) stereoisomer and so these structures
are also enantiomers having the same chemical and physical properties. Each set
of enantiomers is called a diastereomer. Diastereomers are not mirror images of
each other and are completely different compounds having different physical and
chemical properties. To conclude, threonine has two asymmetric centers which
means that there are two possible diastereomers consisting of two enantiomers
each, making a total of four stereoisomers. As the number of asymmetric centers
increases, the number of possible stereoisomers and diastereomers increases.
For a molecule having n asymmetric
centers, the number of possible stereoisomers is 2n and the number of diastereomers is 2n-1.
Related Topics