Chapter: Obstetrics and Gynecology: Cervical Neoplasia and Carcinoma
Prevention of Cervical Neoplasia and Carcinoma
PREVENTION
Preventive approaches to cervical
cancer include sexual abstinence, the use of barrier protection with or without
spermicides, regular gynecologic examination and cyto-logic screening with
treatment of precancerous lesions according to established protocols, and
vaccination with the HPV vaccine. It is estimated that gynecologic exami-nation
and Pap tests administered according to current guidelines may reduce cancer
incidence and mortality by 40%. Limiting the number of sexual partners also may
decrease one’s risk for STDs, including HPV.
The recently developed HPV
vaccine prevents trans-mission and acquisition of type-specific HPV through
sex-ual and nonsexual contact. Currently, the only approved vaccine on the
market is active against oncogenic HPV types 16 and 18 as well as two types
that cause genital warts, HPV types 6 and 11. Another vaccine currently being
investigated is active against oncogenic HPV types 16 and 18. These two
vaccines contain virus-like particles (VLPs) that consist of the main
structural HPV-L1 protein but lack the viral genetic material and, hence, are
non-infectious. These vaccines stimulate production of IgG-type specific
antibodies to prevent acquisition of type specific HPV in the genital and
vulvar areas. The quadrivalent vaccine has been shown to prevent 91% of new and
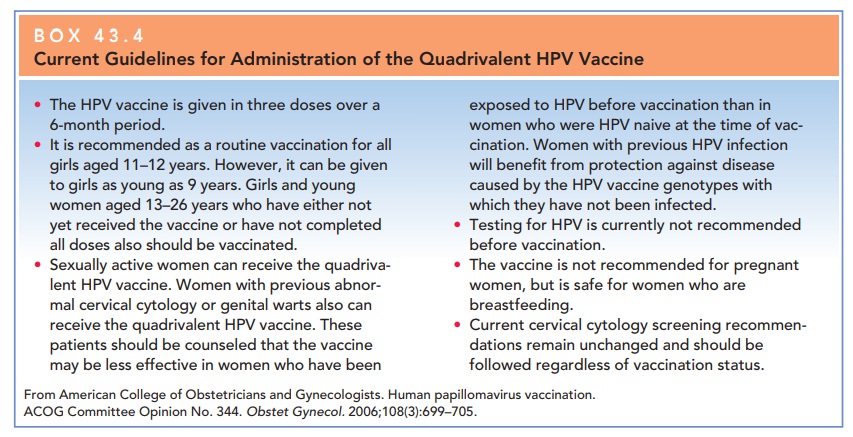
100% of persistent infections. Currently, HPV vaccines are only indicated for
prophylaxis (Box 43-4). However, it is antic-ipated that the guidelines for
their use will continue to change regarding age group, sex, and therapeutic
indica-tions. The development of new vaccines may also broaden the horizon for
HPV treatment.
Related Topics