Chapter: Obstetrics and Gynecology: Cervical Neoplasia and Carcinoma
Management Guidelines for Cervical Epithelial Cell Abnormalities - Cervical Intraepithelial Neoplasia
Management Guidelines for Cervical Epithelial Cell Abnormalities
The American Society for Colposcopy and Cervical Pa-thology (ASCCP) issues guidelines and protocols for the appropriate management of women with cervical cytologic or histologic abnormalities. The most recent updates to these recommendations occurred in 2006 and were published in 2007. These guidelines, including practice algorithms, are available at www.asccp. org/concensus/cytological/shtml. The following sections summarize these guidelines.
LOW-GRADE SQUAMOUS INTRAEPITHELIAL LESIONS AND ATYPICAL SQUAMOUS CELL OF UNDETERMINED SIGNIFICANCE
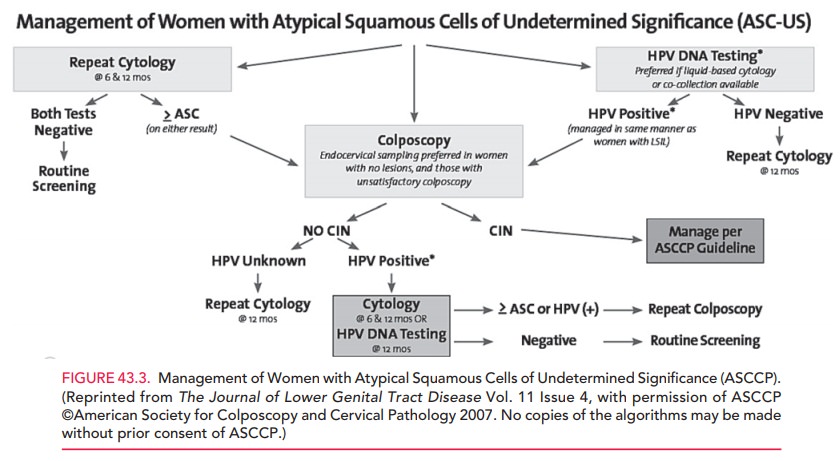
A patient with an ASC-US result
should either undergo HPV DNA testing or repeat cytology at 6 and 12 months
following the abnormal Pap test result. The rationale for HPV DNA testing is
that a negative result obviates the need for colposcopy; patients with ASC-US
who are nega-tive for high-risk HPV DNA may be followed routinely. Women who
screen positive for HPV DNA with ASC-US results on two repeated tests should be
managed in the same way as women with LSIL—both groups should be referred for a
colposcopic evaluation. Patients with ASC-US results at either the 6th- or
12th-month repeat cytology screening should also be referred for colposcopy; if
both repeat tests are negative, then the patient may resume routine screening.
Patients with LSIL are not referred for HPV DNA testing, because 83% of LSIL
patients are positive for HPV, and the test is of little prognostic
significance.
About 3% of Pap test results are
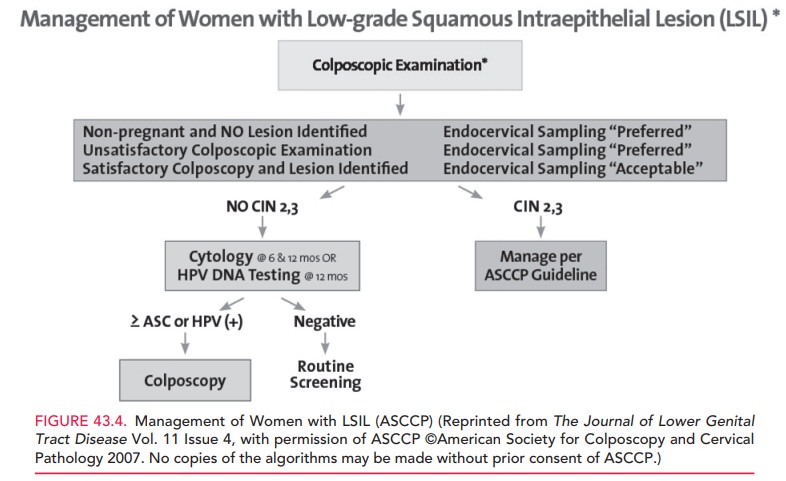
reproducibly classi-fied as LSIL. Management and follow-up are the same
fol-lowing colposcopy for women with LSIL and women with HPV DNA-positive
ASC-US. If no CIN is found, cyto-logic testing is repeated at 6 and 12 months
or HPV DNA testing is repeated at 12 months. A positive result for either ASC
or HPV DNA warrants a repeat colposcopy; women with negative results for ASC or
HPV DNA may resume routine screening (Figs. 43.3 and 43.4).
Management protocols differ for
adolescents and pregnant women. ASC and LSIL are more common in adolescents and
the likelihood of spontaneous regression is higher. Because HPV DNA positivity
is also higher in this population, using HPV DNA screening as a triage method
is not useful. Adolescents with LSIL or ASC-US may be managed with repeat
cytologic testing at 12 months. Those whose repeat test results show HSIL are
referred for colposcopy. Pregnant women with LSIL should not undergo ECC and
should not have more than one col-poscopy during pregnancy. Colposcopic
examination for evaluation of ASC-US can be deferred until at least 6 weeks following
delivery.
Earlier guidelines for postmenopausal women with LSIL Pap test results offered repeat cytologic screening after treatment with vaginal estrogen cream as a triage option, as atrophy of the vaginal mucosa may contribute to the abnormal test result. However, current guidelines recommend that postmenopausal women with LSIL and ASC-US test results be managed in the same way as the general population.
HIGH-GRADE SQUAMOUS INTRAEPITHELIAL LESION (HSIL) AND ATYPICAL SQUAMOUS CELLS— CANNOT EXCLUDE HIGH-GRADE SQUAMOUS INTRAEPITHELIAL LESION (ASC-H)
In the United States, approximately 0.5% of all Pap test results are reported as HSIL. The rate of HSIL decreases with age. CIN 2 or CIN 3 is identified in 84% to 97% of women with HSIL Pap test results, and invasive cancer is identified in 2%. Because the rate of CIN 2 or CIN 3 is so high in adults with HSIL cytologic findings, immediate treatment with the loop electrosurgical excision proce-dure (LEEP; see below) is an acceptable management approach.
In women planning a future
pregnancy, the risks of a LEEP procedure—which include preterm deliv-ery,
premature rupture of membranes, and low-birth-weight infants—should be taken
into consideration. The other management approach is colposcopic examination,
followed by appropriate treatment and follow-up (see Fig. 43.4).
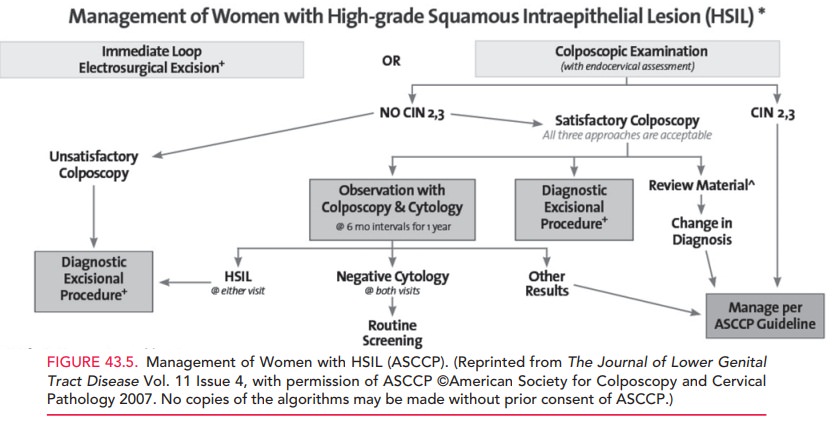
ASC-H is evaluated by colposcopy
because, like HSIL, it carries a higher risk of underlying CIN 2–3 lesions. If
no CIN 2 or CIN 3 is found, the patient may be followed up by repeat screening
at 6 and 12 months or an HPV-DNA test at 12 months. A positive CIN 2, 3, or HPV
DNA result on any of these follow-up tests warrants colposcopic examination; if
results of all follow-up tests are negative, the patient may return to routine
screening (Fig. 43.5).
ATYPICAL GLANDULAR CELLS AND OTHER GLANDULAR ABNORMALITIES
Glandular cell abnormalities
comprise 0.4% of epithelial cell abnormalities. The risk associated with AGC is
dramat-ically higher than that seen with ASC. The risk associated with
glandular abnormalities increases as the description in the Bethesda
classification system advances from AGC—
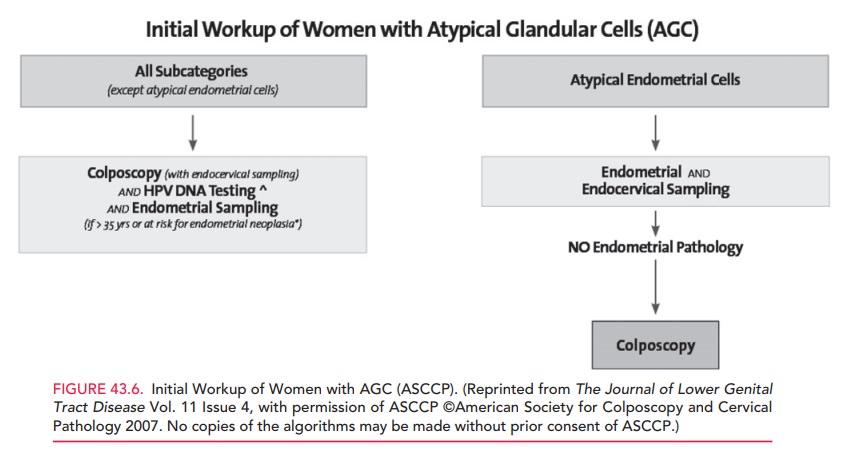
Women with AGC of any type except for atypi-cal endometrial cells should
undergo colposcopic evalua-tion, HPV DNA testing, and ECC. If a woman is older
than 35 years of age or is at risk for endometrial neoplasia (she has
unexplained vaginal bleeding or conditions suggesting chronic anovulation),
endometrial sampling should also be performed. Women with atypical endometrial
cells should have an endometrial biopsy and ECC.
Knowledge of HPV status in women
with AGC who do not have CIN 2 or CIN 3 or glandular neoplasia allows expedited
triage. Women who test positive for HPV at the time of their Pap screening test
should have a repeat Pap test and HPV DNA test at 6 months; those who test
neg-ative for HPV, at 12 months. Women with a positive HPV test and an abnormal
Pap test result should be referred to colposcopy; women who have negative
results on both tests can resume routine screening. In contrast, if HPV status
is not known, the Pap test should be repeated every 6 months until there are
four consecutive negative results, before a woman can resume routine screening
(Fig. 43.6).
Related Topics