Clinical Manifestations, laboratory Diagnosis, Treatment, Prevention and Control - Plasmodium vivax | 12th Microbiology : Chapter 8 : Medical Parasitology
Chapter: 12th Microbiology : Chapter 8 : Medical Parasitology
Plasmodium vivax
Plasmodium vivax
P. vivax shows a similar life cycle in humans and mosquitoes like that of P. falciparum. Except in P. vivax, a latent tissue stage, the hypnozoites present in the liver parenchyma.
Relapse in vivax malaria is caused by these hypnozoites. Hypnozoites are the dormant stages of the parasites. These are single – nucleated parasites measuring 4µm–6µm in diameter. These become active and develop into tissue schizonts after a short period of dormancy. This relapse may occur at intervals up to 3 years or more after the first attack. P. vivax merozoites invade only young erythrocytes and the reticulocytes.
Clinical Manifestations
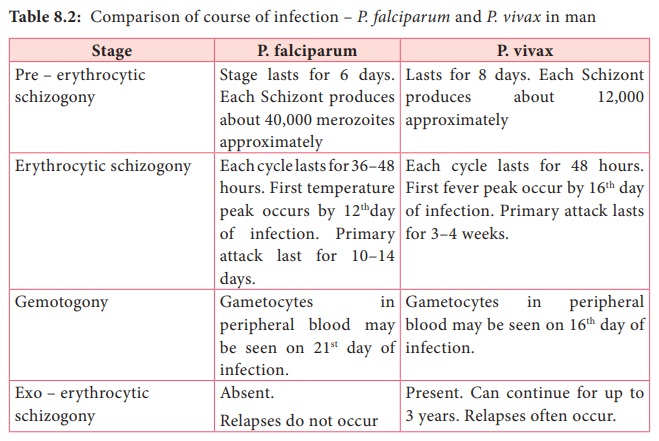
P. vivax is the most wide spread species causing malaria in man. However, unlike Falicparum malariya is less sever and death from the condition relatively is less common table 8.2 describes the comparison of course of infection in falciparum malariya with vivax malaria
laboratory Diagnosis
Diagnosis of malariya includes;
a. Parasitic diagnosis
b. Serodiagnisis and
c. Molecular diagnosis
Parasitic diagnosis – Demonstration of parasite by microscopy
Specimen: Blood
Conventional light microscopy of stained blood smear is the gold standard for confirmation of malaria.
Two types of smears are prepared from the peripheral blood. They are thin and thick smears (Figure 8.14). Ring forms and gametocytes are most commonly seen in the peripheral blood smear.
Thin smear
They are prepared from capillary blood of fingertip and spread over a good quality slide by a second slide (spreader slide) held at an angle of 30°–45° from the horizontal such that a tail is formed
Thin smears thus prepared are air dried, fixed in alcohol and stained by one of the Romanowsky stains such as Leishman, Giemsa or JSB (Jaswant singh and Bhattacharjee) stain.
Thin smears are used for:
a. Detecting parasites, and
b. For determining the species of the infecting parasite.
Thick smear
They are prepared usually with 3 drops of blood spread over a small area of about 10mm. The thick film is dried. This smears consist of a thick layer of dehemoglobinized (lysed) red blood cells. It is not fixed in methanol.
Thick film is stained similar to thin film. Thick smears have the advantage of concentrating the parasites and therefore increase the sensitivity of diagnosis. Thick smears are used for
a. Defecting parasites,
b. Quantitating parasitaemia, an
c. Demonstrating malarial pigments.
Fluoroscence microscopy
The method is mainly used for mass screening in field laboratory. Fluorescent dyes like acridine orange is used to stain the blood smears. It stains DNA as fluorescent green and cytoplasmic RNA as red.
QBC (Quantitative Buffy coat smear)
This is a sensitive method for detection of malaria parasites. In this method, blood is collected in a capillary tube coated with fluorescent dye and is subjected to centrifugation. After centrifugation, the Buffy coat in the centrifuged capillary tubes is examined under a fluorescent microscope. Acridine orange – stained malaria parasites appear brilliant green.
Serodiagnosis
It is not helpful in clinical diagnosis. It is used mainly for epidemiological survey and to identify the infected donors in transfusion malaria. The test used are indirect haemagglutination (IHA), Indirect fluorescent antibody (IFA) and Enzyme – linked immunosorbent assay (ELISA) for the detection of serum antibodies.
Rapid Antigen detection tests kits are available commercially like the dipstick, card and cassette bearing monoclonal antibody. These tests are based on the detection of antigens using immune chromatographic methods. These tests can detect plasmodium in 15 minutes.
Molecular diagnosis
DNA probe and PCR are highly sensitive methods for the diagnosis of malaria. It is more sensitive than that of thick blood smear. It is highly species specific.
Other tests includes the measurement of hemoglobin, total WBC and platelet count in severe falciparum malaria, urine can be tested for free hemoglobin, if black water fever is suspected. Blood urea and serum creatinine has to be monitored for renal failure.
Treatment
The most commonly used drugs are Chloroquine, Quinine, Pyrimethamine and Doxycycline.
Prevention and Control
The preventive measures to control malaria mainly depend on treatment of infected individuals and reducing the transmission of malaria.
The control measures include the use of insecticides such as DDT (Di chlorodiphenyl tri chloromethane) or Malathion for controlling the populations of adult mosquitoes.
Proper use of mosquito nets, wearing protective clothings and use of mosquito repellants can prevent the mosquito bite.
Introduction to Helminths
General characteristics of Helminthic parasite:
1. Helminths are multicellular worms. They are bilaterally symmetrical animals having 3 germ layers and belong to the kingdom Metazoa.
2. They are invertebrates characterised by elongated, flat or round bodies.
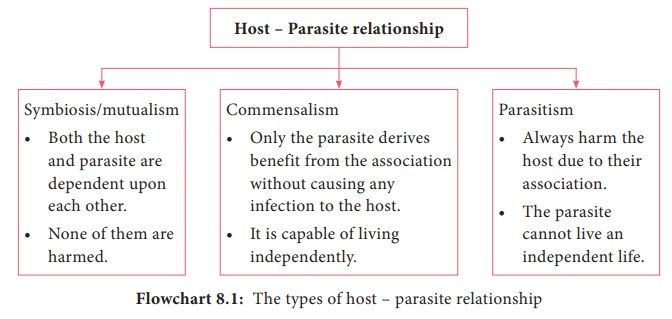
3. Helminths develop through egg, larval and adult stages. Flowchart 8.1 describes the classification of helminthes.
Related Topics