Chapter: Plant Biochemistry: Mitochondria are the power station of the cell
Plant mitochondria have special metabolic functions
Plant mitochondria have special metabolic functions
The function of the mitochondria as being the power station of the cell applies for all mitochondria, from unicellular organisms to animals and plants. In plant cells which perform photosynthesis, the role of the mito-chondria as a supplier of energy is not restricted to the dark phase; the mitochondria provide the cytosol with ATP also during photosynthesis.
Plant mitochondria fulfill additional functions. The mitochondrial matrix contains enzymes for the oxidation of glycine to serine, an impor-tant step in the photorespiratory pathway:
2 glycine + NAD+ + H2O → serine + NADH + CO2 + NH4+
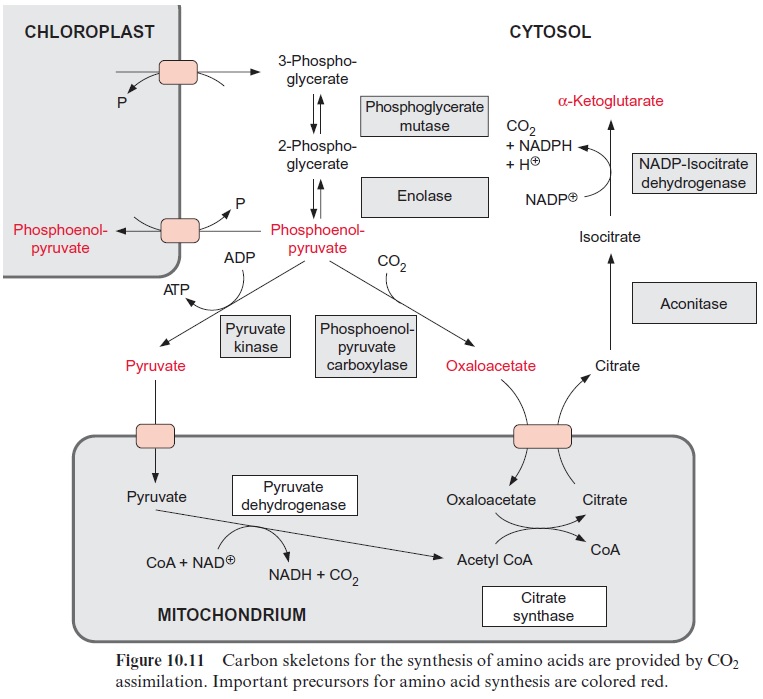
The NADH generated from glycine oxidation is the main fuel for mito-chondrial ATP synthesis during photosynthesis. Another important role of plant mitochondria is the conversion of oxaloacetate and pyruvate to form citrate, a precursor for the synthesis of α-ketoglutarate. This pathway is important for providing the carbon skeletons for amino acid synthesis dur-ing nitrate assimilation (Fig. 10.11).
Mitochondria can oxidize surplus NADH without forming ATP
In mitochondrial electron transport, the participation of flavins, ubisemi-quinones, and other electron carriers leads to the formation of superox-ide radicals, H2O2, and hydroxyl radicals (summarized as ROS, reactive oxygen species ) as by-products. These by-products cause severe cell damage. Since the formation of ROS is especially high, when the components of the respiratory chain are highly reduced, there is a necessity to avoid an overreduction of the respiratory chain. On the other hand, it is essential for a plant that glycine, formed in large quantities by the photores-piratory cycle , is converted by mitochondrial oxidation even when the cell does not require ATP. Plant mitochondria have several over-flow mechanisms, which oxidize surplus NADH without synthesizing ATP in order to prevent an overreduction of the respiratory chain (Fig. 5.21). Among those are an alternative NADH-dehydrogenase, an alternative oxi-dase (Fig. 5.21) and uncoupling proteins. The alternative NADH dehydro-genase, located in the inner mitochondrial membrane, transfers electrons from NADH to ubiquinone, without coupling to proton transport. This pathway is not inhibited by rotenone. However, oxidation of NADH via this rotenone-insensitive pathway proceeds only when the NADH/NAD ratio in the matrix is exceptionally high. In addition, the matrix side of the mitochondrial inner membrane contains an alternative NADPH dehydro-genase (not shown in Figure 5.21).
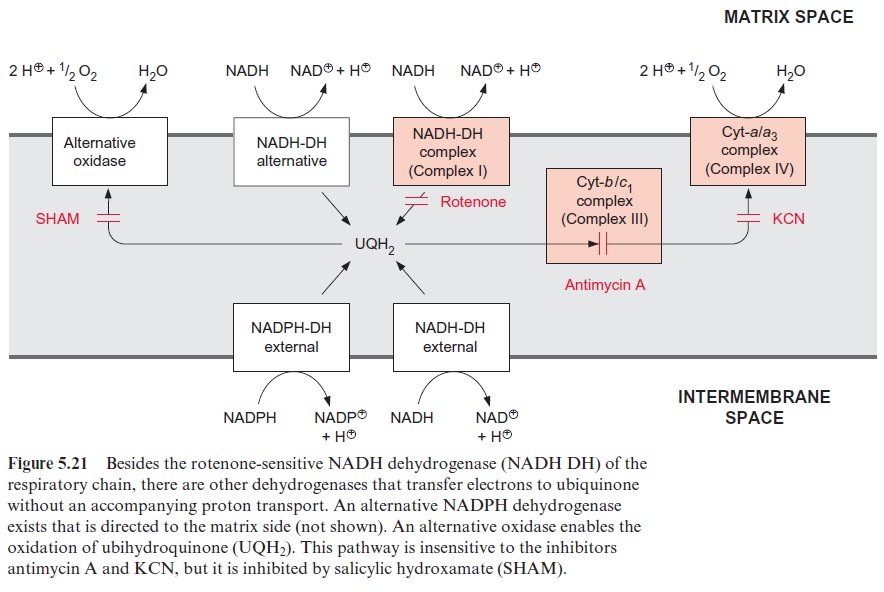
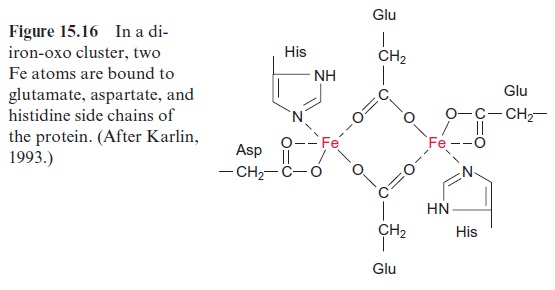
The alternative oxidase transfers electrons directly from ubiquinone to oxygen; this pathway is also not coupled to proton transport. The alterna-tive oxidase is insensitive to antimycin-A and KCN (inhibitors of complex III and II, respectively), but is inhibited by salicylic hydroxamate (SHAM). Recent results show that the alternative oxidase is a membrane protein consisting of two identical subunits (each 36 kDa). From the amino acid sequence it can be predicted that each subunit possesses two transmem-brane helices. The two subunits together form a di-iron oxo-center (like in the fatty acid desaturase, Fig. 15.16), which catalyzes the oxidation of ubi-quinone by oxygen. Electron transport via the alternative oxidase can be understood as a short circuit. It occurs only when the mitochondrial ubiq-uinone pool is highly reduced. The alternative oxidase is activated by a high concentration of pyruvate which is a signal for an excess of metabolites.
Mitochondrial uncoupling proteins were first detected in animal tissues. These proteins are closely related to the mitochondrial ATP/ADP transloca-tor. They build a channel in the inner mitochondrial membrane which is per-meable to protons, resulting in the elimination of the membrane potential, and therefore in the uncoupling of electron transport from ATP synthesis. Uncoupling proteins are widely distributed in eukaryotes; thus also in plants, where they are called PUMPs (plant uncoupling mitochondrial pro-teins). Their apparent function is the prevention of excessive increase of the mitochondrial membrane potential, in order to minimize the formation of reactive oxygen species (ROS).
When metabolites in the mitochondria are in excess, the interplay of the alternative NADH dehydrogenase, the alternative oxidase and the uncou-pling proteins lead to their elimination by oxidation without accompanying ATP synthesis, and the oxidation energy is dissipated as heat. The capacity of the alternative oxidase in the mitochondria from different plant tissues is variable and also depends on the developmental state. Thus one observes a high expression of PUMPs in plants that have been subjected to a cold stress. An especially high alternative oxidase activity has been found in the spadix of the voodoo lily Sauromatum guttatum, which uses the alternative oxidase to heat up the spadix by which volatile amine compounds are emit-ted, which produce a nasty smell like carrion or dung. This strong stench attracts insects from far and wide. The formation of the alternative oxidase is synchronized in these spadices with the beginning of flowering.
NADH and NADPH from the cytosol can be oxidized by the respiratory chain of plant mitochondria
In contrast to mitochondria from animal tissues, plant mitochondria can also oxidize cytosolic NADH and in some cases cytosolic NADPH. Oxidation of this external NADH and NADPH proceeds via two specific dehydrogenases of the inner membrane, of which the substrate binding site is directed towards the intermembrane space. As in the case of succinate dehydrogenase, the electrons from external NADH and NADPH dehy-drogenase are fed into the respiratory chain at the site of ubiquinone, and therefore this electron transport is not inhibited by rotenone. As oxidation of external NADH and NADPH (like the oxidation of succinate) does not involve a proton transport by complex I (Fig. 5.21), the oxidation of external pyridine nucleotides yields less ATP than the oxidation of NADH provided from the matrix. Oxidation by external NADH dehydrogenase proceeds only when the cytosolic NAD system is reduced excessively. Also, the external NADH dehydrogenase may be regarded as part of an overflow mechanism, which comes into action only when the NADH in the cytosol is overreduced. In certain situations photosyn-thesis may produce a surplus of reducing power, which is hazardous for a cell. The plant cell has the capacity to eliminate excessive reducing power by making use of the uncoupling protein PUMP, the external NADH dehydrogenase, the alternative dehydrogenase for internal NADH from the matrix.
Related Topics