Chapter: Plant Biochemistry: Mitochondria are the power station of the cell
Degradation of substrates applicable for biological oxidation takes place in the matrix compartment
Degradation of substrates applicable for biological oxidation takes place in the matrix compartment
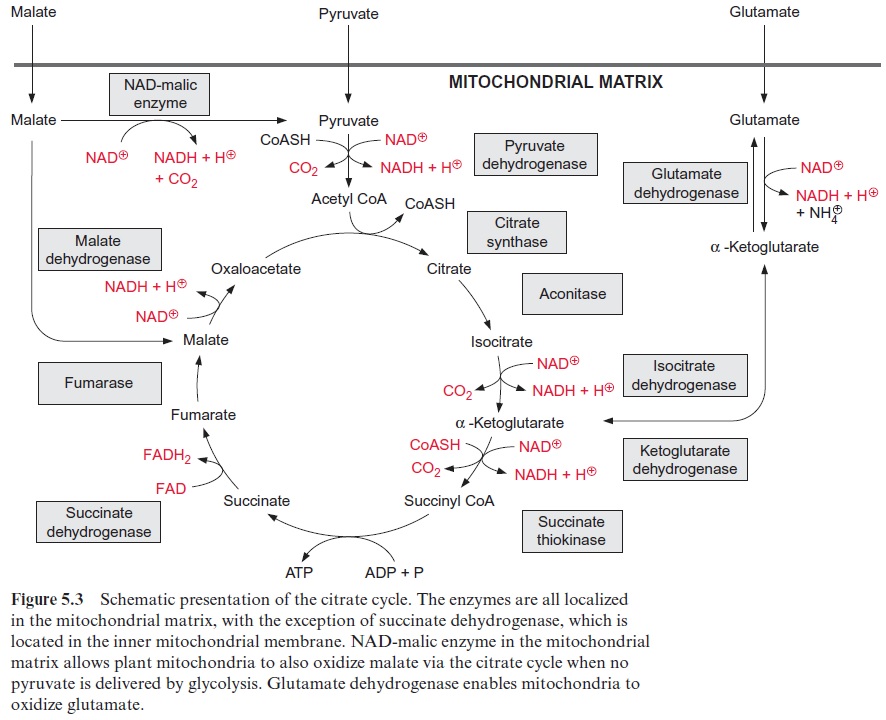
Pyruvate, which is synthesized by the glycolytic catabolism of carbohy-drates in the cytosol, is the starting compound for substrate degradation by the citrate cycle (Fig. 5.2). Pyruvate is first oxidized to acetate (in the form of acetyl coenzyme A), which is then completely degraded to CO2 by the citrate cycle, yielding 10 reducing equivalents [H] to be oxidized by the respiratory chain to generate ATP. Figure 5.3 shows the reactions of the citrate cycle.
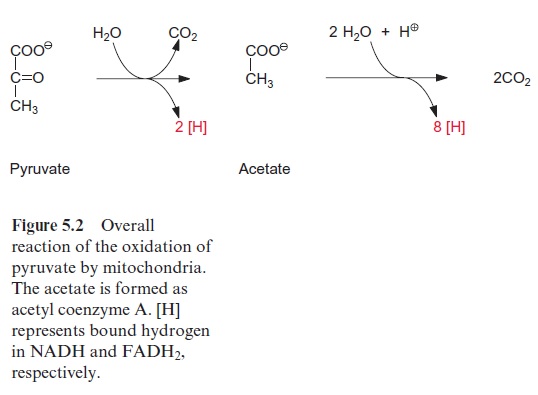
Pyruvate is oxidized by a multienzyme complex
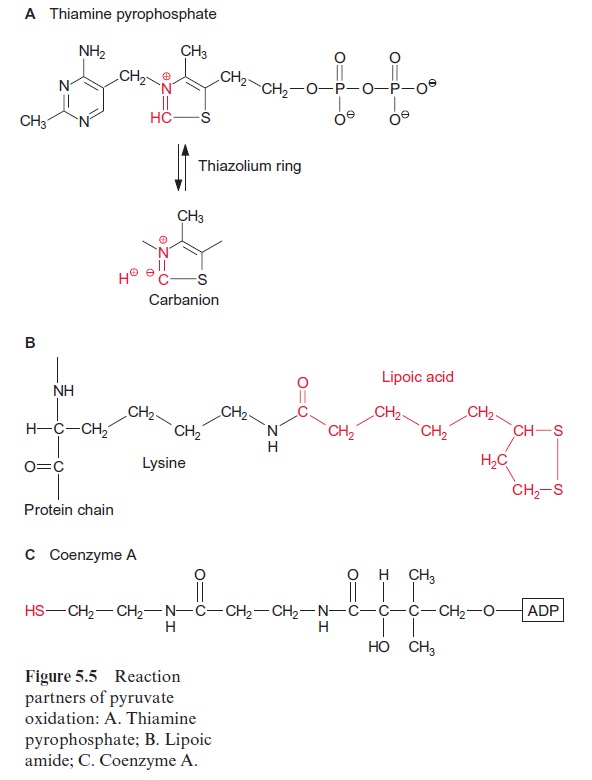
Pyruvate oxidation is catalyzed by the pyruvate dehydrogenase complex, a multienzyme complex located in the mitochondrial matrix. It consists of three different catalytic subunits: pyruvate dehydrogenase, dihydrolipoyl transacetylase, and dihydrolipoyl dehydrogenase (Fig. 5.4). The pyruvate dehydrogenase subunit containsthiamine pyrophosphate (TPP, Fig. 5.5A) as the prosthetic group. The reactive group of TPP is the thiazole ring. Due to the presence of a positively charged N atom, the thiazole ring contains an acidic C-atom. After dissociation of a proton, a carbanion is formed, which binds to the carbonyl group of the pyruvate. The positively charged N atom of the thiazole ring enhances the decarboxylation of the bound pyruvate and hydroxethyl-TPP is formed (Fig. 5.4). The hydroxethyl group is now transferred to lipoic acid.
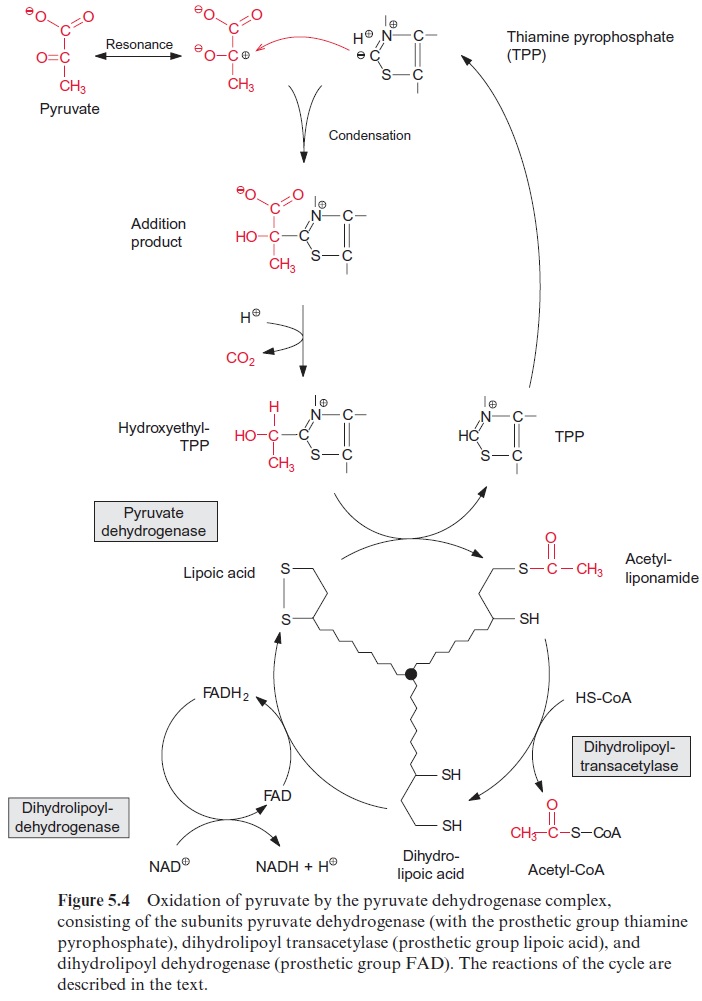
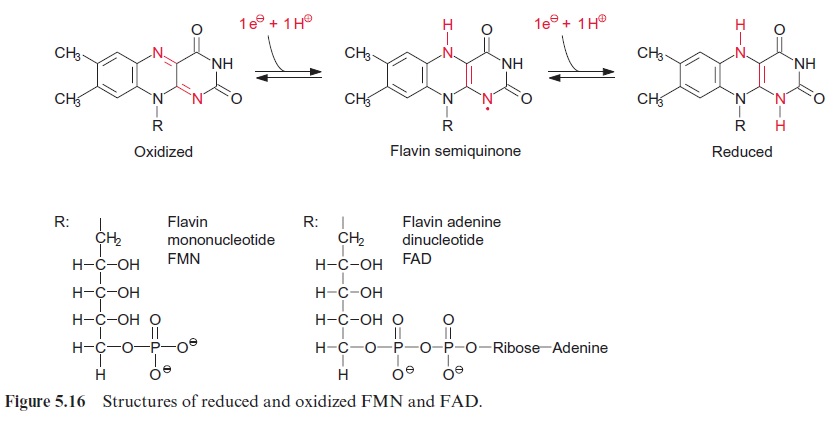
Lipoic acid is the prosthetic group of the dihydrolipoyl transacetylase subunit. It is covalently bound by its carboxyl group to a lysine residue of the enzyme protein via an amide bond (Fig. 5.5B). The lipoic acid resi-due is attached to the protein by a long carbon chain and therefore able to react with the various reaction sites of the multienzyme complex. Lipoic acid is equipped with two S atoms linked by a disulfide bond. When the hydroxyethyl residue is transferred to the lipoic acid residue, lipoic acid is reduced to dihydrolipoic acid and the hydroxyethyl residue is oxidized to an acetyl residue. The latter is now attached to the dihydrolipoic acid by a thioester bond. Thioester bonds are rich in energy, and store the energy released during oxidation of the carbonyl group. The acetyl residue is then transferred by dihydrolipoyl transacetylase to the sulfhydryl group of coen-zyme A (Fig. 5.5C) to synthesize acetyl coenzyme A. Acetyl CoA—also called active acetic acid—was discovered by Feodor Lynen from Munich (1984 Nobel Prize in Medicine). Dihydrolipoic acid is reoxidized to lipoic acid by dihydrolipoyl dehydrogenase and NAD+ is reduced to NADH via FAD (see Fig. 5.16). A pyruvate dehydrogenase complex is also present in the chloroplasts.
Acetate is completely oxidized in the citrate cycle
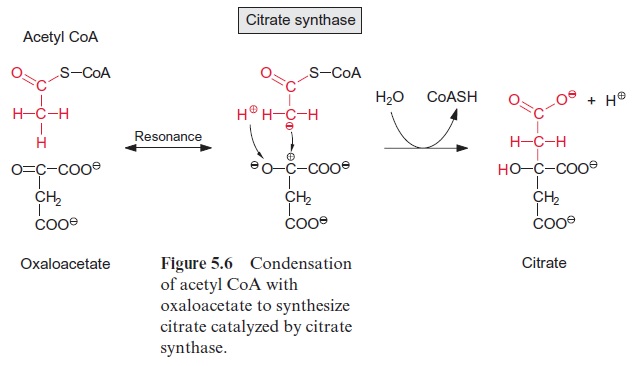
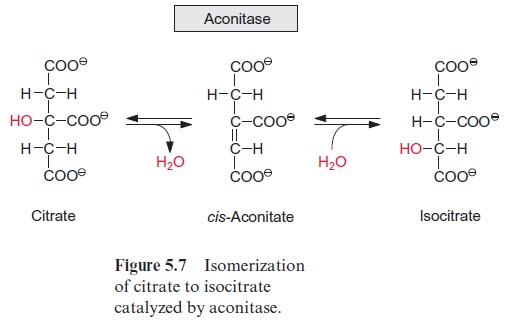
Acetyl coenzyme A enters the citrate cycle and condenses with oxaloacetate to citrate (Fig. 5.6). This reaction is catalyzed by the enzyme citrate syn-thase. The energy of the thioester group promotes the removal of a proton of the acetyl residue, and the carbanion thus formed binds to the carbo-nyl carbon of oxaloacetate. Subsequent release of CoA-SH makes the reac-tion irreversible. The enzyme aconitase (Fig. 5.7) catalyzes the reversible isomerization of citrate to isocitrate. In this reaction, first water is released, and the cis-aconitate thus formed remains bound to the enzyme and is then isomerized to isocitrate by the addition of water. In addition to the mito-chondrial aconitase, there is also an isoenzyme of aconitase in the cytosol of plant cells.
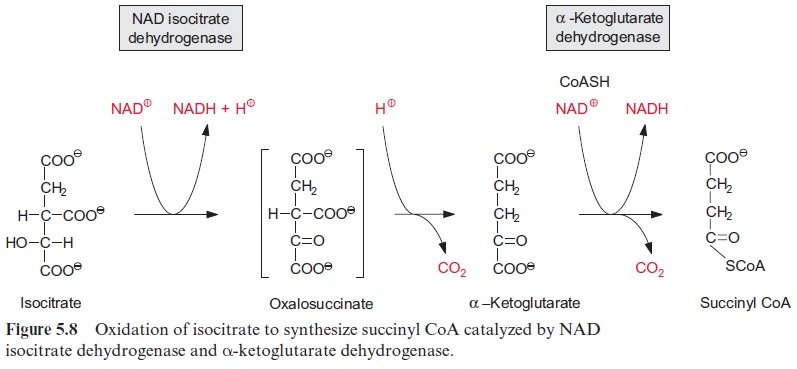
Oxidation of isocitrate to α-ketoglutarate by NAD isocitrate dehydro-genase (Fig. 5.8) results in the formation of NADH. Oxalosuccinate is formed as intermediate, tightly bound to the enzyme to be decarboxylated to α-ketoglutarate (also termed 2-oxo-glutarate). This oxidative decar-boxylation is an irreversible reaction. Besides the NAD-isocitrate dehydro-genase, mitochondria also contain an NADP-isocitrate dehydrogenase.
NADP-isocitrate dehydrogenases also occur in the chloroplast stroma and in the cytosol.
Oxidation of α-ketoglutarate to succinyl-CoA (Fig. 5.8) is catalyzed by the α -ketoglutarate dehydrogenase multienzyme complex. This complex contains thiamine pyrophosphate, lipoic acid, and FAD, analogously to the pyruvate dehydrogenase multienzyme complex which catalyzes the reaction of pyruvate to acetyl CoA.
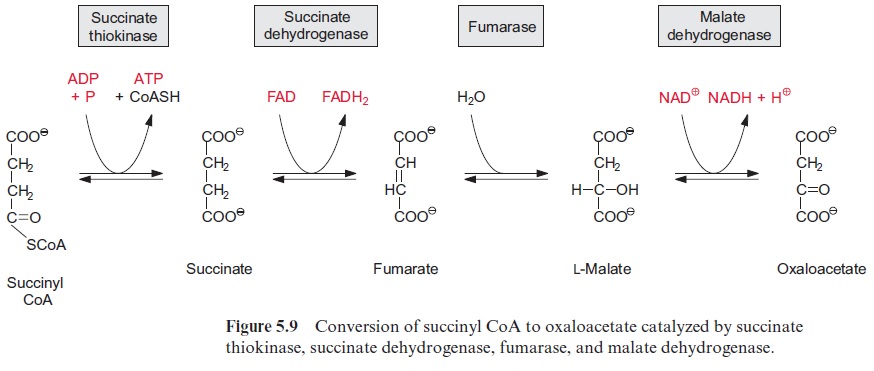
The thioester bond of the succinyl CoA is rich in energy. In the succi-nate thiokinase reaction, the free energy released upon the hydrolysis of this thioester is utilized to form ATP (Fig. 5.9). It may be noted that in animal metabolism the mitochondrial succinate thiokinase reaction yields GTP. The succinate formed is oxidized by succinate dehydrogenase to syn-thesize fumarate. Succinate dehydrogenase is the only enzyme of the citrate cycle that is not located in the matrix, but in the mitochondrial inner mem-brane, with its succinate binding site accessible from the matrix . Reducing equivalents (FADH2) derived from succinate oxidation are transferred to ubiquinone. Catalyzed by fumarase, water reacts by trans-addition with the C-C double bond of fumarate to form L-malate. This is a reversible reaction (Fig. 5.9). Oxidation of malate by malate dehydrogenase, yielding oxaloacetate and NADH, is the final step in the citrate cycle (Fig. 5.9). The reaction equilibrium of this reversible reaction favors strongly the educt malate.
[NADH] . [ oxaloacetate] / [NAD+] . [malata] = 2.8 . 10-5 (pH 7)
Due to this equilibrium reaction, it is essential for an efficient operation of the citrate cycle that the citrate synthase reaction is irreversible. In this way oxaloacetate can be withdrawn from the malate dehydrogenase equi-librium to further support the reactions of the citrate cycle. Isoenzymes of malate dehydrogenase also occur outside the mitochondria. Both the cytosol and the peroxisomal matrix contain NAD-malate dehydrogenases, while an NADP-malate dehydrogenase is present only in the chloroplast stroma.
A loss of intermediates of the citrate cycle is replenished by anaplerotic reactions
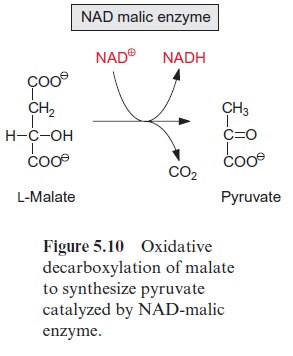
The citrate cycle can proceed only when the oxaloacetate required as accep-tor for the acetyl residue is fully regenerated. It is necessary, therefore, to replenish the loss of citrate cycle intermediates by anaplerotic reactions. In contrast to mitochondria from animal tissues, plant mitochondria are able to transport oxaloacetate into the chloroplasts via a specific translocator of the inner membrane . Therefore, the citrate cycle can be replenished by the uptake of oxaloacetate, which has been synthesized by phosphoenolpyruvate carboxylase in the cytosol. Oxaloacetate can also be delivered by oxidation of malate in the mitochondria. Malate is stored in the vacuole and is an important substrate for mitochondrial respiration. A special feature of plant mitochondria is that malate is oxidized in the matrix via NAD-malic enzyme to pyruvate with the reduction of NAD+ and the release of CO2 (Fig. 5.10). Thus interplay of malate dehydrogenase and NAD-malic enzyme allows citrate to be formed from malate without the operation of the complete citrate cycle (Fig. 5.3). It may be noted that an NADP-dependent malic enzyme is present in the chloroplasts, especially in C4plants.

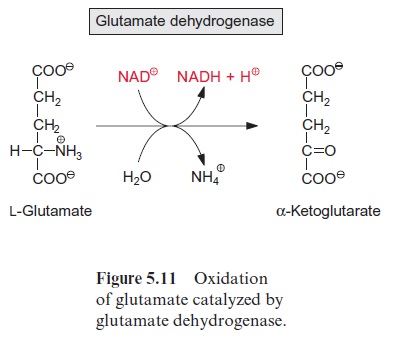
Another important substrate of mitochondrial oxidation is glutamate, which is one of the main products of nitrate assimilation and, besides sucrose, the most highly concentrated organic compound in the cytosol of many plant cells. Glutamate oxidation, accompanied by for-mation of NADH, is catalyzed by glutamate dehydrogenaselocated in the mitochondrial matrix (Fig. 5.11). This enzyme also reacts with NADP+ . NADP-glutamate dehydrogenase activity is also present in plastids, although its function is yet not understood.
Glycine is the main substrate of respiration in the mitochondria from mesophyll cells of illuminated leaves.
Related Topics