Chapter: Plant Biochemistry: Mitochondria are the power station of the cell
Electron transport of the respiratory chain is coupled to the synthesis of ATP via proton transport
Electron transport of the respiratory chain is coupled to the synthesis of ATP via proton transport
The electron transport of the respiratory chain is coupled to the forma-tion of ATP. This is illustrated in the experiment of Figure 5.19, in which the velocity of respiration in a mitochondrial suspension was determined by measuring the decrease of the oxygen concentration in the suspension medium. The addition of a substrate alone (e.g., malate) causes only a minor increase in respiration. The subsequent addition of a limited amount of ADP results in a considerable acceleration of respiration. After some time, however, respiration returns to the lower rate prior to the addition of ADP, as the ADP has been completely converted to ATP. Respiration in the pres-ence of ADP is called active respiration, whereas that after ADP is consumed is called controlled respiration. As the ADP added to the mitochondria is completely converted to ATP, the amount of ATP formed with the oxida-tion of a certain substrate can be determined from the ratio of ADP added to oxygen consumed (ADP/O). An ADP/O of about 2.5 is determined for substrates oxidized in the mitochondria via the formation of NADH (e.g., malate), and of about 1.6 for succinate, from which the redox equivalents are directly transferred via FADH to ubiquinone.
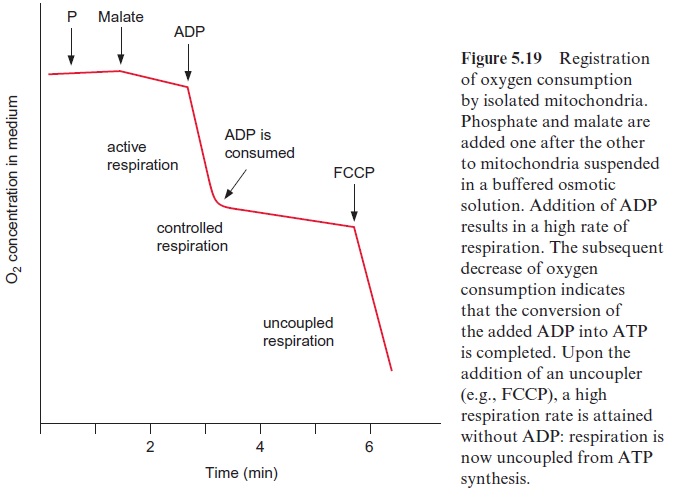
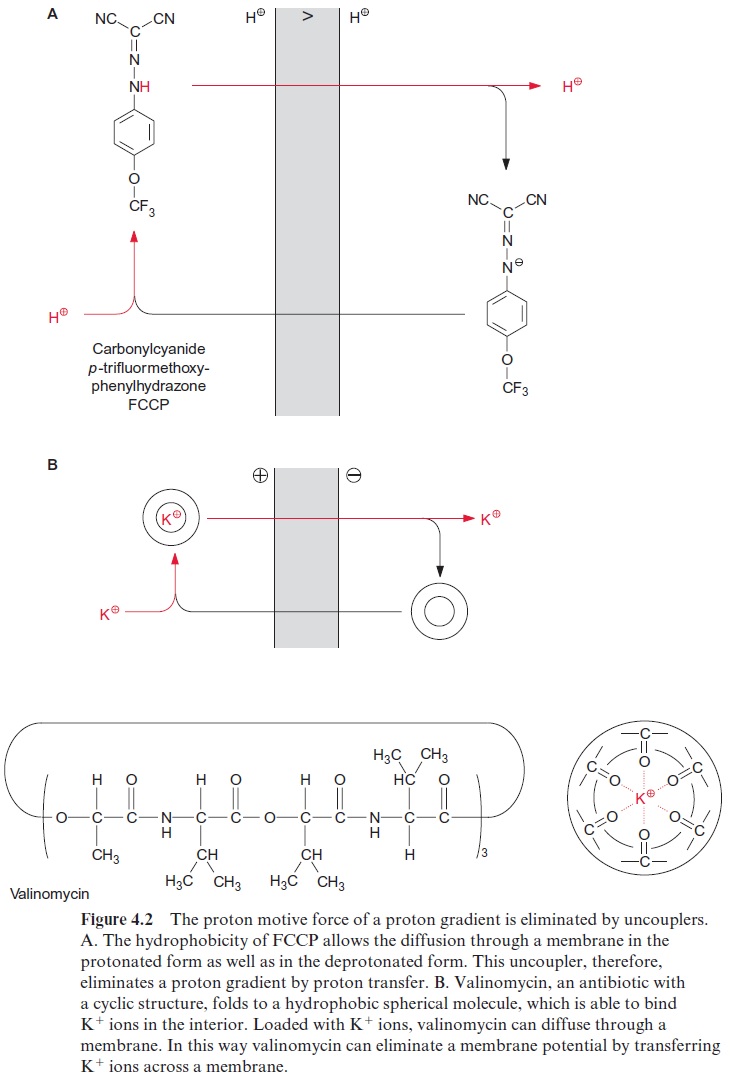
Like photosynthetic electron transport , the electron trans-port of the respiratory chain is accompanied by the generation of a proton motive force (Fig. 5.15), which in turn drives the synthesis of ATP (Fig. 5.20). Therefore substances such as FCCP (Fig. 4.2) function as uncouplers of mitochondrial as well as photosynthetic electron transport.Figure 5.19 shows that the addition of the uncoupler FCCP results in a high stimula-tion of respiration. The uncoupling function of the FCCP is due to a short circuit of protons across a membrane, resulting in the elimination of the proton gradient. The respiration is then uncoupled from ATP synthesis and the energy set free during electron transport is dis-sipated as heat.
To match respiration to the energy demand of the cell, it is regulated by an overlapping of two different mechanisms. The classic mechanism of respiratory control is based on the fact that when the ATP/ADP ratio increases, the proton motive force also increases, which in turn causes a decrease of electron transport by the respiratory chain. Recently it was dis-covered that ATP also impedes the electron transport by binding to a subu-nit of cytochrome oxidase, which results in a decrease of its activity.
Mitochondrial proton transport results in the formation of a membrane potential
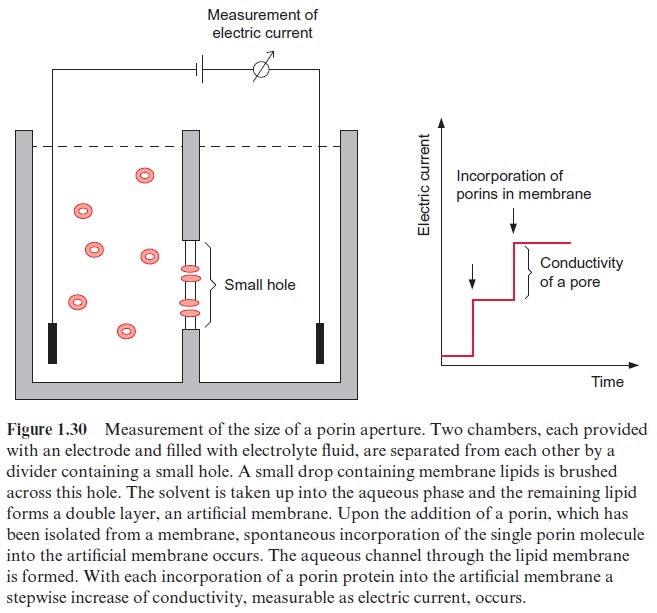
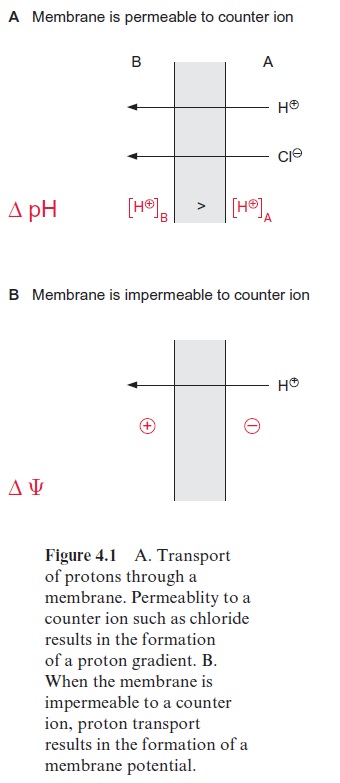
Mitochondria, in contrast to chloroplasts, have no closed thylakoid space to form a proton gradient. Instead, in mitochondrial electron transport, protons are transported from the matrix to the intermembrane space, which is, however, connected to the cytosol by pores (formed by porines (Fig. 1.30)). In chloroplasts the formation of a proton gradient of ΔpH 2.5 in the light results in a decrease of pH in the thylakoid lumen from about pH 7.5 to pH 5.0. If during mitochondrial oxidation such a strong acidification were to occur in the cytosol, it would have a grave effect on the activity of the cytosolic enzymes. In fact, during mitochondrial controlled respiration the ΔpH across the inner membrane is only about 0.2, and therefore mito-chondrial proton transport leads primarily to the formation of a membrane potential ( ΔΨ≈200 mV). Mitochondria are unable to generate a larger pro-ton gradient, as their inner membrane is impermeable for anions, such as chloride. As shown in Figure 4.1, a proton concentration gradient can be formed only when the charge of the transported protons is compensated by the diffusion of a counter anion.
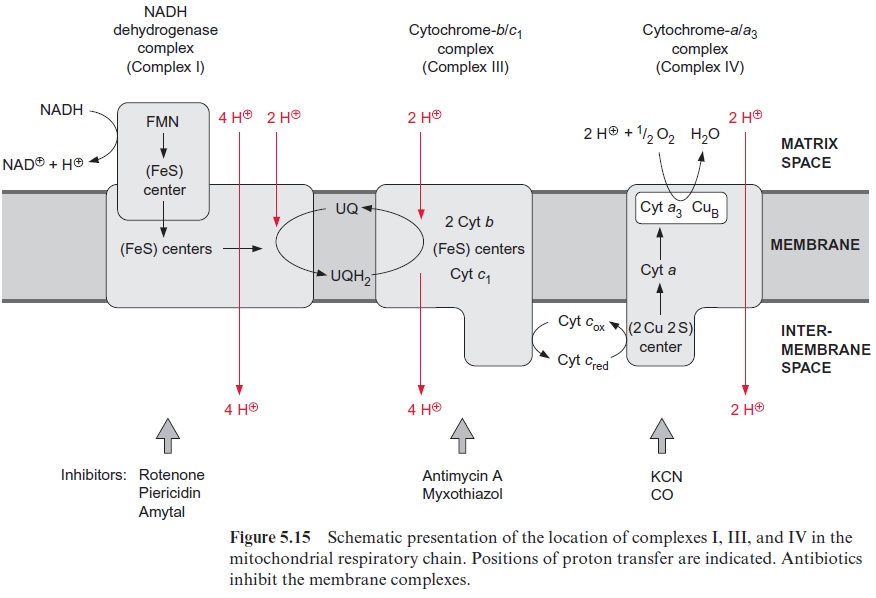
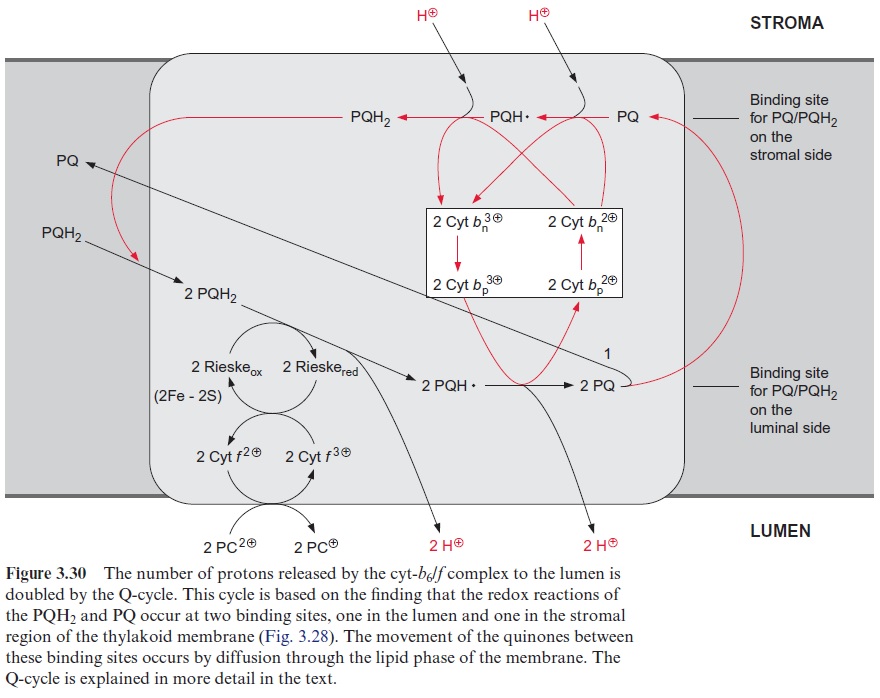
Despite intensive research for more than 30 years our knowledge of the mechanism of coupling between mitochondrial electron transport and transport of protons is still incomplete. Four protons are probably taken up from the matrix side during the transport of two electrons from the NADH dehydrogenase complex to ubiquinone and released into the intermembrane space by the cyt-b/c1 complex (Fig. 5.15). It is generally accepted that in mitochondria the cyt-b/c1 complex catalyzes a Q-cycle (Fig. 3.30) by which, when two electrons are transported, two additional protons are transported out of the matrix space into the intermembrane space. Finally, the cyt-a/a3complex transports two protons per two electrons. The three-dimensional structure of the cyt-a/a3 complex indicates that the binuclear center from cytochrome-a3 and CuBis involved in this proton transport. If these stoichi-ometries are correct, altogether 10 protons would be transported during the oxidation of NADH and only six during the oxidation of succinate.
Mitochondrial ATP synthesis serves the energy demand of the cytosol
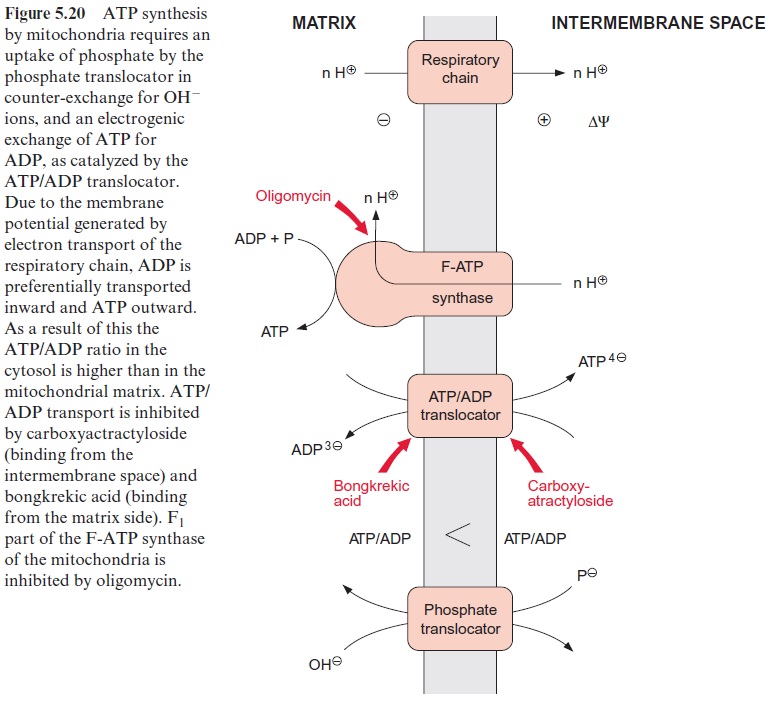
The energy of the proton gradient is used in the mitochondria for ATP syn-thesis by an F-ATP synthase (Fig. 5.20), which has the same basic struc-ture as the F-ATP synthase of chloroplasts . However, there are differences regarding the inhibition by oligomycin, an antibiotic from Streptomyces. Whereas the mitochondrial F-ATP synthase is very strongly inhibited by oligomycin, due to the presence of an oligomycin binding pro-tein, the chloroplast enzyme is insensitive to this inhibitor. Although the mechanism of ATP synthesis appears to be identical for both ATP syn-thases, the proton stoichiometry in mitochondrial ATP synthesis has not been resolved unequivocally. Assuming that the rotor of the F-ATP syn-thase in mitochondria has 10 c-subunits, 3.3 protons would be required for the synthesis of 1 mol of ATP (according to the mechanism for ATP syn-thesis). This rate corresponds more or less with pre-vious independent investigations.
In contrast to chloroplasts, which synthesize ATP essentially for their own consumption, the ATP in mitochondria is synthesized mainly for export into the cytosol. This requires the uptake of ADP and phosphate from the cytosol into the mitochondria and vice versa the release of the syn-thesized ATP. The uptake of phosphate proceeds by the phosphate translo-cator in a counter-exchange for OH- ions, whereas the uptake of ADP and the release of ATP are mediated by the ATP/ADP translocator (Fig. 5.20). The mitochondrial ATP/ADP translocator is inhibited by carboxyatracty-loside, a glucoside from the thistle Atractylis gumnifera, and by bongkrekic acid, an antibiotic from the bacterium Cocovenerans, growing on coconuts. Both compounds are deadly poisons.
The ATP/ADP translocator catalyzes a strict counter-exchange; for each ATP or ADP transported out of the chloroplasts, an ADP or ATP is trans-ported inward. Since the transported ATP contains one negative charge more than the ADP, the transport is electrogenic. Due to the membrane potential generated by the proton transport of the respiratory chain, there is a preference for ADP to be taken up and ATP to be transported out-ward. As a result of this asymmetric transport of ADP and ATP the ATP/ ADP ratio outside the mitochondria is much higher than in the matrix. In this way mitochondrial ATP synthesis maintains a high ATP/ADP ratio in the cytosol. With the exchange of ADP for ATP, one negative charge is transferred from the matrix to the outside, which requires the transport of a proton in the other direction to compensate this charge difference. This is why protons from the proton gradient are consumed not only for ATP synthesis as such, but also for export of the synthesized ATP from the mitochondria.
Let us return to the stoichiometry between the transported protons and the ATP formation during respiration. It is customary to speak of three coupling sites of the respiratory chain, which correspond to the complexes I, III, and IV. Textbooks often state that during NADH oxidation by the mitochondrial respiratory chain, one molecule of ATP is formed per cou-pling site, and as a result of this, the ADP/O quotient for oxidation of NADH amounts to three, and that for succinate to two. However, con-siderably lower values have been determined in experiments with isolated mitochondria. The attempt was made to explain this discrepancy by assum-ing that owing to a proton leakage of the membrane, the theoretical ADP/ O values were not attained in the isolated mitochondria. It appears now that even in theory these whole numbers for ADP/O ratios are incorrect. Probably 10 protons are transported upon the oxidation of NADH. In the event that 3.3 protons are required for the synthesis of ATP and another one for its export from the mitochondria, the resulting ADP/O would be 2.3. With isolated mitochondria, values of about 2.5 have been obtained experimentally.
The change in free energy during the oxidation of NADH was evaluated to be -214 kJ/mol and for the synthe-sis of ATP as about +50 kJ/mol. An ADP/O of 2.3 for the respiration of NADH-dependent substrates indicates that about 54% of the free energy released during oxidation is used for the synthesis of ATP. However, these values must still be treated with caution.
Related Topics