Chapter: Plant Biochemistry: Mitochondria are the power station of the cell
How much energy can be gained by the oxidation of NADH?
How much energy can be gained by the oxidation of NADH?
How much energy is released during mitochondrial respiration or, to be more exact, how large is the difference in free energy in the mitochondrial redox processes? To answer this question the differences of the potentials of the redox pairs are calculated by the Nernst equation:
E = E0’ + [RT / nF] ln [oxidized substance / reduced substance]
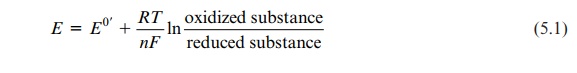
where E0 standard potential at pH 7, 25°C; R (gas constant) 8.31 J/ K · mol; T 298 K; n is the number of electrons transferred; and F(Faraday constant) 96,485 A s/mol.
The standard potential for the redox pair NAD+ /NADH is:
E0’ = -0.320 V
Under certain metabolic conditions, an NAD+ /NADH ratio was found to be 3 in mitochondria from leaves. The introduction of this value into equation 5.1 yields:
ENAD+/NADH = -0.320 + RT / 2F ln3 = -0.306 V
The standard potential for the redox pair H2O/O2 is:
E0’ = +0.815 V ([H2O] in water 55 mol/L)
To evaluate the actual potential of [O2] the partial pressure of the oxy-gen in the air is introduced:
EH20/02 = 0.815 + RT / 2F ln √P O2
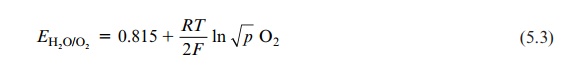
The partial pressure of the oxygen in the air (pO2) is 0.2. Introducing this value into equation 5.3 yields:
EH20/02 = 0.805 V
The difference of the potentials amounts to:
ΔE = EH20/02 - ENAD+/NADH = +1.11 V
The free energy ( ΔG) is related to ΔE as follows:
ΔG = - nFΔE (5.5)
Two electrons are transferred in the reaction. The introduction of E into equation 5.5 shows that the change of free energy during the oxidation of NADH by the respiratory chain amounts to:
ΔG = -214 kJ/mol
How much energy is required for the formation of ATP? It has been calculated that the synthesis of ATP under the meta-bolic conditions in the chloroplasts requires a change of free energy of ΔG≈+50 kJ/mol. This value also applies approximately for the ATPwhich mitochondria provide for the cytosol.
The calculated free energy released by the oxidation of NADH would therefore be sufficient to generate four molecules of ATP, but in fact the amount of ATP synthesized by NADH oxidation in vivo is much lower .
Related Topics