Chapter: Clinical Anesthesiology: Anesthetic Management: Maternal & Fetal Physiology & Anesthesia
Physiology of Normal Labor
PHYSIOLOGY OF NORMAL LABOR
On average, labor commences 40 ± 2 weeks follow-ing the last menstrual
period. The factors involved in the initiation of labor likely involve
distention of the uterus, enhanced myometrial sensitivity to oxy-tocin, and
altered prostaglandin synthesis by fetal membranes and decidual tissues.
Although circu-lating oxytocin levels often do not increase at the beginning of
labor, the number of myometrial oxy-tocin receptors rapidly increases. Several
prodromal events usually precede true labor approximately 2–4 weeks prior to
delivery: the fetal presenting part settles into the pelvis (lightening);
patients develop uterine (Braxton Hicks) contractions that are
char-acteristically irregular in frequency, duration, and intensity; and the
cervix softens and thins out (cervi-cal effacement). Approximately 1 week to 1
h before true labor, the cervical mucous plug (which is often bloody) breaks
free (bloody show).
True labor begins when the sporadic Braxton
Hicks contractions increase in strength (25–60 mm Hg), coordination, and
frequency (15–20 min apart). Amniotic membranes may rupture spontaneously prior
or subsequent to the onset of true labor. Following progressive cervical
dilation, the con-tractions propel first the fetus and then the placenta
through the pelvis and perineum. By convention, labor is divided into three
stages. The first stage is defined by the onset of true labor and ends with
complete cervical dilation. The second stage begins with full cervical
dilation, is characterized by fetal descent, and ends with complete delivery of
the fetus. Finally, the third stage extends from the birth of the baby to the
delivery of the placenta.
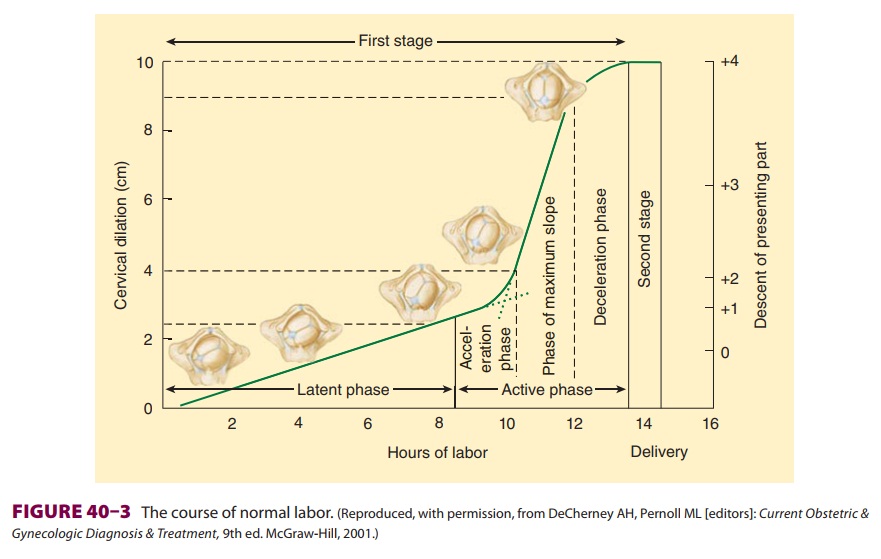
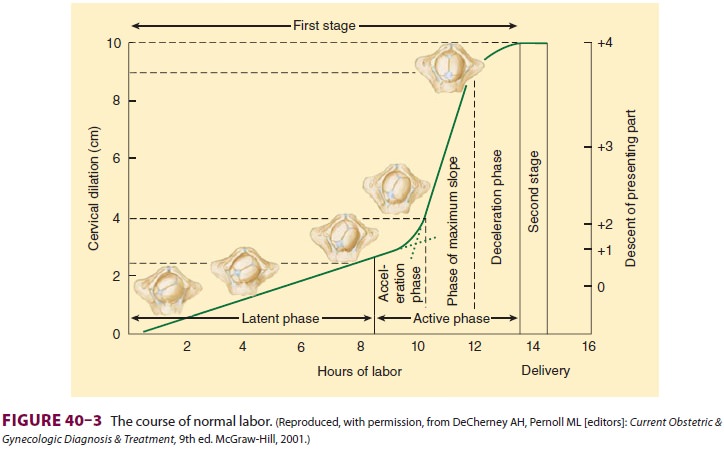
Based on the rate of cervical dilation, the first stage is further
divided into a slow latent phase fol-lowed by a faster active phase (Figure
40–3). The latent phase is characterized by progressive cervical effacement
and minor dilation (2–4 cm). The subse-quent active phase is characterized by
more frequent contractions (3–5 min apart) and progressive cervi-cal dilation
up to 10 cm. The first stage usually lasts 8–12 h in nulliparous patients and
about 5–8 h in multiparous patients.
Contractions during the second stage occur 1.5–2 min apart and last
1–1.5 min. Although con-traction intensity does not appreciably change, the
parturient, by bearing down, can greatly augment intrauterine pressure and
facilitate expulsion of the fetus. The second stage usually lasts 15–120 min
and the third stage typically 15–30 min.
The course of labor is monitored by uterine activity, cervical dilation,
and fetal descent. Uterine activity refers to the frequency and magnitude of
uterine contractions. The latter may be measured directly, with a catheter
inserted through the cer-vix, or indirectly, with a tocodynamometer applied
externally around the abdomen. Cervical dilation and fetal descent are assessed
by pelvic examination. Fetal station refers to the level of descent (in
centi-meters) of the presenting part relative to the ischial spines (eg, –1 or +1).
Effect of Labor on Maternal Physiology
During intense painful contractions, maternal min-ute ventilation may
increase up to 300%. Oxygen consumption also increases by an additional 60%
above third-trimester values. With excessive hyper-ventilation, Paco2 may
decrease below 20 mm Hg. Marked hypocapnia can cause periods of
hypoven-tilation and transient maternal and fetal hypoxemia between
contractions. Excessive maternal hyper-ventilation also reduces uterine blood
flow and pro-motes fetal acidosis.
Each contraction places an additional burden on the heart by displacing
300–500 mL of blood from the uterus into the central
circulation (analogous to an autotransfusion). Cardiac output rises 45% over
third-trimester values. The greatest strain on the heart, however, occursimmediately
after delivery, when intense uterine contraction and involution suddenly
relieve inferior vena caval obstruction and increase cardiac output as much as
80% above late third trimester values.
Effect of Anesthetic Agents on Uterine Activity & Labor
A. Inhalational Agents
Sevoflurane, desflurane, isoflurane, and halothane depress uterine
activity equally at equipotent doses; all cause dose-dependent uterine
relaxation. Low doses (<0.75 MAC)
of these agents, however, do not interfere with the effect of oxytocin on the
uterus. Higher doses can result in uterine atony and increase blood loss at
delivery. Nitrous oxide has minimal, if any, effects.
B. Parenteral Agents
Opioids minimally decrease the progression of labor; ketamine, in doses
of less than 2 mg/kg, appears to have little effect.
C. Regional Anesthesia
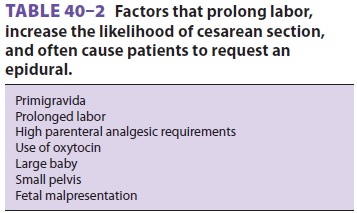
The administration of epidural analgesia is usually based upon the
patient’s choice, and it is often uti-lized for patients with
maternal or fetal factors that increase the
likelihood of prolonged labor or cesarean delivery (Table 40–2). Current evidence indicates that dilute combinations of a localanesthetic
(eg, bupivacaine, 0.125% or less) and an opioid (eg, fentanyl, 5 mcg/mL or
less) for epidural
or combined spinal–epidural (CSE) analgesia do not prolong labor or
increase the likelihood of operative delivery.
When greater concentrations of local
anesthetic (eg, bupivacaine, 0.25%) are used for continuous epidural analgesia,
the second stage of labor may be prolonged by approximately 15–30 min. Intense
regional analgesia/anesthesia can remove the urge to bear down during the
second stage (Ferguson reflex), and motor weakness can impair expulsive
efforts, often prolonging the second stage of delivery. Use of dilute local
anesthetic–opioid mixtures can preserve motor function and allow effective
push-ing. Intravenous fluid loading (crystalloid boluses) is often used to
prevent or reduce the severity of hypo-tension following an epidural injection.
So-called fluid loading does not reduce the incidence of hypotension and has
been shown to reduce endog-enous oxytocin secretion from the pituitary and
transiently decrease uterine activity. Epinephrine-containing local anesthetic
solutions could theoreti-cally prolong the first stage of labor if absorption
of epinephrine from the epidural space results in sig-nificant systemic β-adrenergic effects. Prolongation of labor is
generally not clinically observed with very dilute (eg, 1:400,000)
epinephrine-containing local anesthetics.
D. Vasopressors
Uterine muscle has both α and β receptors. α1-Receptor stimulation causes uterine contraction, whereas β2-receptor stimulation produces
relax-ation. Large doses of α-adrenergic
agents, such as phenylephrine, in addition to causing uterine arte-rial
constriction, can produce tetanic uterine con-tractions. Small doses of
phenylephrine (40 mcg) may increase uterine blood flow in normal parturi-ents
by raising arterial blood pressure. In contrast, ephedrine has little effect on
uterine contractions.
E. Oxytocin
Oxytocin (Pitocin) is usually administered
intrave-nously to induce or augment uterine contractions or to maintain uterine
tone postpartum. It has a half-life of 3–5 min. Induction doses for labor are
0.5–8 mU/min. Complications include fetal dis-tress due to hyperstimulation,
uterine tetany, and, less commonly, maternal water retention (antidiuretic
effect). Rapid intravenous infusion can cause transient systemic hypotension
due to relaxation of vascular smooth muscle; reflex tachycardia may also be
noted.
Uterine atony is the most common cause of
severe postpartum hemorrhage. Immediate admin-istration of oxytocin after
delivery is a standard measure to prevent this complication. Despite this
practice, uterine atony complicates 4–6% of pregnan-cies. The concentration of
volatile anesthetics should be reduced to 0.5 MAC in obstetric patients
undergo-ing general anesthesia for cesarean delivery to avoid the
uterine-relaxing effects of these drugs. Second-line oxytocics are
methylergonovine (Methergine) and carboprost tromethamine (Hemabate).
F. Ergot Alkaloids
Methylergonovine (Methergine) causes intense and prolonged uterine
contractions. It is therefore given only after delivery (postpartum) to treat
uterine atony. Moreover, because it also constricts vascular smooth muscle and
can cause severe hypertension if given as an intravenous bolus, it is usually
admin-istered only as a single 0.2 mg dose intramuscularly or in dilute form as
an intravenous infusion over 10 minutes.
G. Prostaglandins
Carboprost tromethamine (Hemabate,
prostaglan-din F2α) is a synthetic analogue of prostaglandin F2 that stimulates uterine contractions. It is
often used to treat refractory postpartum hemorrhage. An ini-tial dose of 0.25
mg intramuscularly may be repeated every 15–90 min to a maximum of 2 mg. Common
side effects include nausea, vomiting, broncho-constriction, and diarrhea. It
is contraindicated in patients with bronchial asthma. Prostaglandin E1 (Cytotec, rectal suppository) or E 2 (Dinoprostone, vaginal suppository) is
sometimes administered and has no bronchoconstricting effect.
H. Magnesium
Magnesium is used in obstetrics both to stop pre-mature labor
(tocolysis) and to prevent eclamptic seizures. It is usually administered as a
4 g intra-venous loading dose (over 20 min) followed by a 2 g/h infusion.
Therapeutic serum levels are consid-ered to be 6–8 mg/dL. Serious side effects
include hypotension, heart block, muscle weakness, and sedation. Magnesium in
these doses and concentra-tions intensifies neuromuscular blockade from
non-depolarizing agents.
H. β2 Agonists
The β2-adrenergic agonists ritodrine
and terbutaline inhibit uterine contractions and are used to treat premature
labor.
Related Topics