Chapter: Clinical Anesthesiology: Anesthetic Management: Maternal & Fetal Physiology & Anesthesia
Anesthesia for Uteroplacental Circulation
UTEROPLACENTAL CIRCULATION
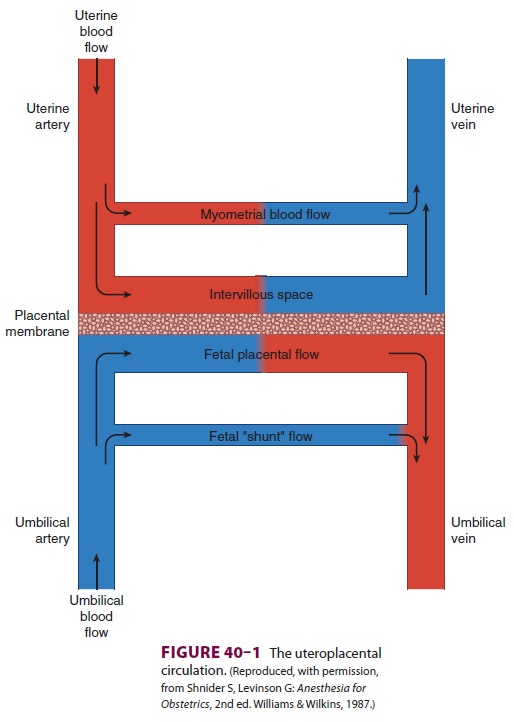
A normal uteroplacental circulation ( Figure
40–1) is critical in the development and
maintenance of a healthy fetus. Uteroplacental insufficiency is an important
cause of intrauterine fetal growth retarda-tion, and when severe, can result in
fetal demise. The integrity of this circulation is, in turn, dependent on both
adequate uterine blood flow and normal pla-cental function.
Uterine Blood Flow
At term, uterine blood flow represents about
10% of the cardiac output, or 600–700 mL/min (compared with 50 mL/min in the
nonpregnant uterus). Eighty percent of uterine blood flow normally supplies the
placenta; the remainder goes to the myometrium. Pregnancy maximally dilates the
uterine vascula-ture, so that autoregulation is absent, but the uterine
vasculature remains sensitive to α-adrenergic ago-nists. Uterine blood flow is not usually significantly
affected by respiratory gas tensions, but extreme hypocapnia (Paco2<20 mm Hg) can reduce
uterine blood flow and causes fetal hypoxemia and acidosis.
Blood flow is directly proportionate to the
dif-ference between uterine arterial and venous pres-sures but inversely
proportionate to uterine vascular resistance. Although not under appreciable
neural control, the uterine vasculature has α-adrenergic and possibly some β-adrenergic receptors.
Three major factors decrease uterine blood flow during pregnancy: (1)
systemic hypotension, uterine
vasoconstriction, and (3) uterine contrac-tions. Common causes of hypotension
during preg-nancy include aortocaval compression, hypovolemia, and sympathetic
blockade following regional anes-thesia. Stress-induced release of endogenous
cate-cholamines (sympathoadrenal activation) during labor causes uterine
arterial vasoconstriction. Any drug with α-adrenergic activity (eg, phenylephrine) potentially is capable of
decreasing uterine blood
flow by vasoconstriction. Ephedrine, whichhas considerable β-adrenergic activity, has traditionally been considered the vasopressor of choice for hypotension during pregnancy. However, clinical studies suggest that α-adrenergic agonists such as phenylephrine and metaraminol are just as effective in treating hypotension in pregnant patients and are associated with less fetal acidosis than ephedrine.Paradoxically, hypertensive disorders are often associated with decreased uterine blood flow due to generalized vasoconstriction. Uterine contractions decrease uterine blood flow by elevating uterine venous pressure and compressing arterial ves-sels as they traverse the myometrium. Hypertonic contractions during labor or during oxytocin infu-sions can critically compromise uterine blood flow.
Placental Function
The fetus is dependent on the placenta for
respira-tory gas exchange, nutrition, and waste elimination. The placenta is
formed by both maternal and fetal tissues and derives a blood supply from each.
The resulting exchange membrane has a functional area of about 1.8 m2.
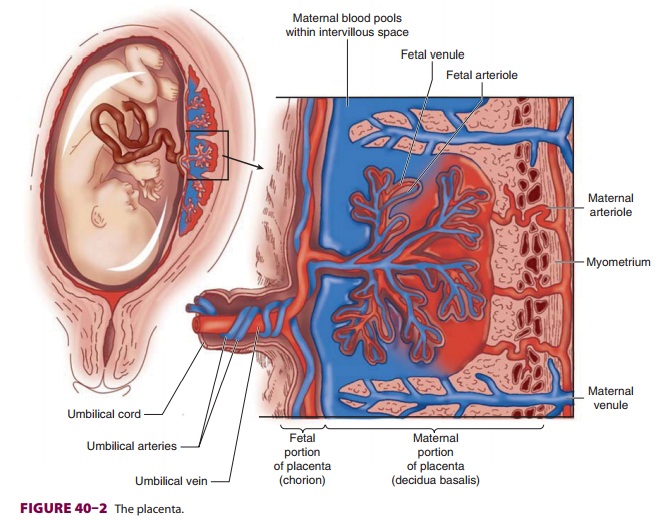
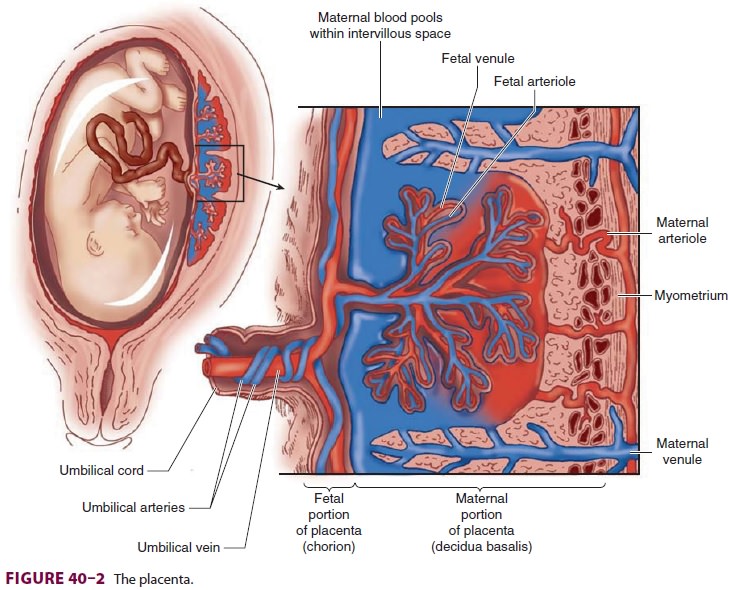
A. Physiological Anatomy
The placenta (Figure 40–2) is composed of projec-tions of fetal tissue (villi) that lie in
maternal vascular spaces (intervillous spaces). As a result of this
arrange-ment, the fetal capillaries within villi readily exchange substances
with the maternal blood that bathes them. Maternal blood in the intervillous
spaces is derived from spiral branches of the uterine artery and drains into
the uterine veins. Fetal blood within villi is derived from the umbilical cord
via two umbilical arteries and returns to the fetus via a single umbilical
vein.
B. Placental Exchange
Placental exchange can occur by one of six mecha-nisms:
· Diffusion—Respiratory gases and small ionsare transported by diffusion. Most drugs used in anesthesia have molecular weights well under 1000 and consequently can readily diffuse across the placenta.
·
Osmotic and hydrostatic pressure (bulk flow)—Water
moves across by osmotic and hydrostatic pressures. Water enters the fetal
circulation in quan-tities greater than any other substance.
·
Facilitated diffusion—Glucose enters the
fetalcirculation down the concentration gradient (no energy is consumed)
facilitated by a specific trans-porter molecule.
·
Active
transport—Amino acids, vitamin B12, fattyacids, and some ions
(calcium and phosphate) uti-lize this mechanism.
·
Vesicular transport—Large molecules, such
asimmunoglobulins, are transported by pinocytosis.
·
Iron enters the fetal circulation in
this way, facili-tated by ferritin and transferrin.
·
Breaks—Breaks in the placental
membrane maypermit mixing of maternal and fetal blood. This probably underlies
Rh sensitization . Rh sensitization occurs most commonly during delivery.
Respiratory Gas Exchange
At term, fetal oxygen consumption averages about 7 mL/min per kilogram
of fetal body weight. Fortunately, because of multiple adaptive mecha-nisms,
the normal fetus at term can survive 10 min or longer instead of the expected 2
min in a state of total oxygen deprivation. Partial or complete oxygen
deprivation can result from umbilical cord compression, umbilical cord
prolapse, placental abruption, severe maternal hypoxemia, or hypoten-sion.
Compensatory fetal mechanisms include redis-tribution of blood flow primarily
to the brain, heart, placenta, and adrenal gland; decreased oxygen
con-sumption; and anaerobic metabolism.
Transfer of oxygen across the placenta is
depen-dent on the ratio of maternal uterine blood flow to fetal umbilical blood
flow. The reserve for oxygen transfer is small even during normal pregnancy.
Normal fetal blood from the placenta has a Pao2 of only 30–35 mm Hg. To aid oxygen transfer, the fetal hemoglobin
oxygen dissociation curve is shifted to the left such that fetal hemoglobin has
greater affinity for oxygen than does maternal hemoglobin (whose curve is
already shifted to the right; see the section on Respiratory Effects). In
addition, fetal hemoglo-bin concentration is usually 15 g/dL (compared with
approximately 12 g/dL in the mother).
Carbon dioxide readily diffuses across the
pla-centa. Maternal hyperventilation (see the section on Respiratory Effects)
increases the gradient for the transfer of carbon dioxide from the fetus into
the maternal circulation. Fetal hemoglobin has less affin-ity for carbon
dioxide than do adult forms of hemo-globin. Carbon monoxide readily diffuses across
the placenta, and fetal hemoglobin has greater affinity for carbon monoxide
than do adult forms.
Placental Transfer of Anesthetic Agents
Transfer of a drug across the placenta is reflected by the ratio of its
fetal umbilical vein to maternal venous concentrations (UV/MV), whereas its
uptake by fetal tissues can be correlated with the ratio of its fetal umbilical
artery to umbilical vein concentra-tions (UA/UV). Fetal effects of drugs
administered to parturients depend on multiple factors, including route of
administration (oral, intramuscular, intra-venous, epidural, or intrathecal),
dose, timing of administration (both relative to delivery as well as
contractions), and maturity of the fetal organs (brain and liver). Thus, a drug
given hours before delivery or as a single intravenous bolus during a uterine
contraction just prior to delivery (when uterine blood flow is maximally
reduced) is unlikely to pro-duce high fetal levels. Fortunately, current
anesthetic techniques for labor and delivery generally have minimal fetal
effects despite significant placental transfer of anesthetic agents and
adjuncts.
All inhalational agents
and most intravenous agents freely cross the placenta. Inhalational
agentsgenerally produce little fetal depression when they are given in limited
doses (<1 MAC) and delivery occurs within 10 min of induction. Ketamine,
pro-pofol, and benzodiazepines readily cross the pla-centa and can be detected
in the fetal circulation. Fortunately, when these agents (with the excep-tion
of benzodiazepines) are administered in usual induction doses, drug
distribution, metabolism, and possibly placental uptake may limit fetal
effects. Although most opiates readily cross the placenta, their effects on
neonates at delivery vary consider-ably. Newborns appear to be more sensitive
to the respiratory depressant effect of morphine compared with other opioids.
Although meperidine produces respiratory depression, peaking 1–3 h after
admin-istration, it produces less than morphine; butorpha-nol and nalbuphine
produce even less respiratory depression but still may have significant
neurobe-havioral depressant effects. Although fentanyl read-ily crosses the
placenta, it appears to have minimal neonatal effects unless larger intravenous
doses (>1 mcg/kg) are given immediately before delivery. Epidural or intrathecal
fentanyl, sufentanil, and, to a lesser extent, morphine, generally produce
minimal neonatal effects. Alfentanil causes neonatal depres-sion similar to
meperidine. Remifentanil also readily crosses the placenta and has the
potential to produce respiratory depression in newborns. Fetal blood
con-centrations of remifentanil are generally about half those of the mother
just prior to delivery. The UA/ UV ratio is about 30%, suggesting fairly rapid
metab-olism of remifentanil in the neonate. The highly ion-ized nature of
muscle relaxants impedes placental transfer, resulting in minimal effects on
the fetus.
Local anesthetics are weakly basic drugs that are
principally bound to α1-acid glycoprotein.
Placental transfer depends on three factors: (1) pKa , (2) maternal and fetal pH, anddegree of protein
binding. Except for chloropro-caine, fetal acidosis increases fetal-to-maternal
drug ratios because binding of hydrogen ions to the non-ionized form causes
trapping of the local anestheticin the fetal circulation. Highly protein-bound
agents diffuse slowly across the placenta; thus, greater pro-tein binding of
bupivacaine and ropivacaine, com-pared with that of lidocaine, likely accounts
for their lower fetal blood levels. Chloroprocaine has the least placental
transfer because it is rapidly broken down by plasma cholinesterase in the
maternal circulation.
Most commonly used anesthetic adjuncts also readily
cross the placenta. Thus, maternally admin-istered ephedrine, β-adrenergic blockers
(such as labetalol and esmolol), vasodilators, phenothiazines, antihistamines
(H1 and H2), and metoclopramide are transferred to the
fetus. Atropine and scopolamine, but not glycopyrrolate, cross the placenta;
the latter’s quaternary ammonium (ionized) structure results in only limited
transfer.
Effect of Anesthetic Agents on Uteroplacental Blood Flow
Intravenous anesthetic agents have variable
effects on uteroplacental blood flow. Propofol and barbitu-rates are typically
associated with small reductions in uterine blood flow due to mild to moderate,
dose-dependent decreases in maternal blood pressure. A small induction dose,
however, can produce greater reductions in blood flow as a result of sympathoad-renal
activation (due to light anesthesia). Ketamine in doses of less than 1.5 mg/kg
does not appreciably alter uteroplacental blood flow; its hypertensive effect
typically counteracts any vasoconstriction. Uterine hypertonus may occur with
ketamine at doses of more than 2 mg/kg. Etomidate likely has minimal effects,
but its actions on uteroplacental cir-culation have not been well-described.
Volatile
inhalational anesthetics decrease blood pressure and, potentially,
uteroplacental blood flow. In concentrations of less than 1 MAC, however, their
effects are generally minor, consist-ing of dose-dependent uterine relaxation
and minorreductions in uterine blood flow. Nitrous oxide has minimal effects on
uterine blood flow when adminis-tered with a volatile agent. In animal studies,
nitrousoxide alone can vasoconstrict the uterine arteries. High blood levels of
local anesthetics—particularly lidocaine—cause uterine arterial
vasocon-striction. Such levels are seen only with unintentional intravascular
injections and occasionally following paracervical blocks (in which the
injection site is in close proximity to the uterine arteries), and local
absorption or injection into these vessels cannot be ruled out). Spinal and
epidural anesthesia typically do not decrease uterine blood flow except when
arte-rial hypotension occurs. Moreover, uterine blood flow during labor may
actually improve in preeclamp-tic patients following epidural anesthesia; a
reduc-tion in circulating endogenous catecholamines likely decreases uterine
vasoconstriction. The addition of dilute concentrations of epinephrine to local
anes-thetic solutions does not appreciably alter uterine blood flow.
Intravascular uptake of the epinephrine from the epidural space may result in
only minor sys-temic β-adrenergic effects.
Related Topics