Chapter: Essentials of Psychiatry: Sleep and Sleep-Wake Disorders
Physiological Regulation of Sleep and Wakefulness
Physiological
Regulation of Sleep and Wakefulness
Three
physiological processes regulate sleep and wakefulness.
Ultradian Rhythm of Rapid Eye Movement (REM) and Non-rapid Eye Movement (Non-REM) Sleep
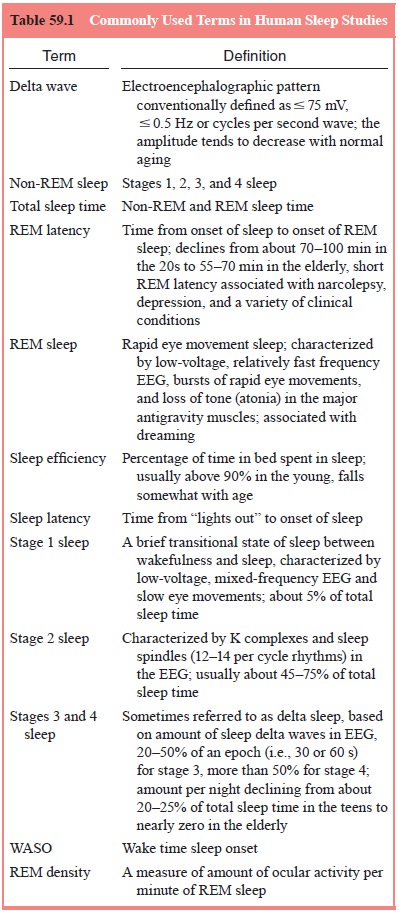
Sleep
consists of two major REM and non-REM sleep, which alternate throughout the
sleep period. Sleep normally begins in the adult with non-REM sleep and is
followed after about 70 to 90 minutes by the first REM period. Thereafter,
non-REM sleep and REM sleep oscillate with a cycle length (the interval
be-tween onset of each non-REM or REM period) of about 80 to 110 minutes. This
cycle of REM and non-REM sleep is an ex-ample of an ultradian rhythm, a
biological rhythm with a cycle length considerably less than 24 hours.
On the
basis of electroencephalographical (EEG) charac-teristics, non-REM sleep in
humans is further divided into four stages: stage 1, a brief transitional stage
between wakefulness and sleep; stage 2, which occupies the greatest amount of
time during sleep; and stages 3 and 4, sometimes called delta sleep be-cause of
the characteristic high-amplitude slow EEG waves (delta waves) (Table 59.1).
The amount of ocular activity per minute of REM sleep is quantified as REM
density; this can be meas-ured by either visual scoring (e.g., on an analogue
scale from 0 to 8 per minute) or by computer analysis. Dreaming is commonly
reported and is usually vivid when subjects are awakened from REM sleep but
also occurs during non-REM sleep, especially at sleep onset during stage 1
sleep.
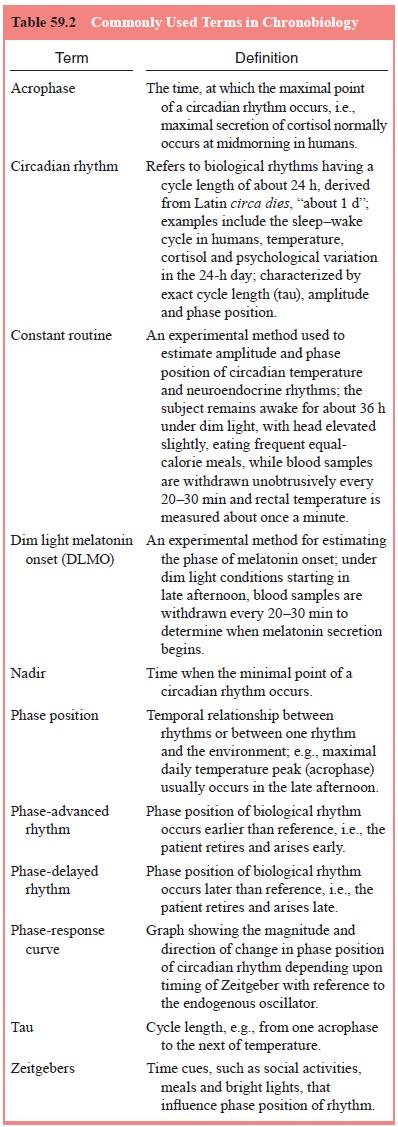
Circadian (24-Hour) Rhythm of Sleep and Wakefulness
The
rest–activity or sleep–wake cycle is an example of a circa-dian rhythm (Table
59.2). Other examples include the hypotha-lamic–pituitary–adrenal axis,
thyroid-stimulating hormone and core body temperature. [Circadian rhythms can
be characterized by three difference measures: 1) cycle length (tau) (e.g., the time between two peaks
of the \sim24 hour temperature curve); 2) am-plitude (e.g., the difference
between the minimum value of the cycle (nadir)
and maximum value (acrophase), for
example, the difference between the lowest and highest points in the 24 hour
temperature curve; and 3) phase position of the rhythm (e.g., the time of day
when the acrophase occurred).]
The
propensity for sleep and wakefulness varies in a cir-cadian fashion, at least
after infancy, and is modulated in part by one or more biological clocks. The
suprachiasmatic nucleus (SCN) in the anterior hypothalamus plays a decisive
role in the regulation of most circadian rhythms in humans and animals. The
endogenous activity rhythms of the SCN are synchronized with the environment
primarily by ambient light. Information re-garding light reaching the retina is
conveyed to the SCN directly through the retinohypothalamic tract and
indirectly through the intergeniculate leaflet of the lateral geniculate body.
Changes in light intensity, especially at dawn and dusk, are particularly
important in synchronizing endogenous oscillators controlling rhythms of
sleep–wake, cortisol, melatonin and core body tem-perature with one another and
with the outside world.
If humans
are allowed to choose their sleep–wake cycles in the absence of time cues such
as daily light–dark signals or clocks, they usually show, as most mammalian
species do, a sleep–wake cy-cle longer than 24 hours. The self-selected
rest–activity cycle is typi-cally about 24.5 to 25 hours in length, although it
may increase, for example, to 36 hours (24 hours awake and 12 hours asleep).
These observations imply that neurons within the SCN have an inherent
rhythmicity of approximately 24.5 to 25 hours. Subjects in a time-free
environment are said to be “free- running” because endogenous processes, such
as a circadian oscillator, rather than environmental cues, determine their
sleep–wake, endocrine and other rhythms.
The
propensity for, character and duration of sleep are closely related to the
phase position of the underlying circadian oscillator. If the daily temperature
curve is used to index the phase position of the biological clock, sleep in
general and REM sleep in particular occur most commonly near the nadir of the
temperature rhythm. Thus, in persons who live a conventional sleep schedule (11
PM–7 AM), REM sleep is more common in the last half of the night when core body
temperature is lowest than in the first half and more likely in morning naps
than in afternoon naps. Furthermore, subjects tend to awaken on the rising
phase of the temperature rhythm.
Appropriate exposure to light and darkness can change the phase position of the underlying biological oscillator or, in some circumstances, the amplitude of circadian rhythms. Bright light at the beginning of the subjective evening in conjunction with dark during the subjective morning delays and resets the phase position of the temperature, cortisol, melatonin and sleep–wake rhythms; dark in the subjective evening and bright light in the subjective morning have the opposite effect. The magnitude and direction of the changes induced by bright light or other Zeitgebers (“timegiv-ers”) at any particular time form the phase-response curve.
The phase position of the circadian oscillator can also be estimated in humans by the 24-hour rhythms of cortisol or melatonin secretion. Because these rhythms can be affected by exercise, meals, light and so forth, the conditions under which they are measured should be controlled. For example, clinical in-vestigators may use a “constant routine” condition in which the subjects are kept awake in bed for 36 hours, under constant low-intensity. An alternative is to determine the onset of melatonin secretion under dark conditions (dim light melatonin onset). As discussed later, various strategies are under experimental development with the hope that appropriate administration of light–dark cycles, melatonin, vitamin B12, or specific medica-tions will “nudge” and “squash” the circadian oscillator cor-rectly better to manage clinical disorders of sleep–wakefulness, such as jet lag, delayed sleep syndrome and shift work. In addi-tion, bright light has been shown to have antidepressant effects in patients with winter depression, some patients with major depressive disorder and patients with premenstrual dysphoric disorder.
If
animals suffer lesions of the SCN, they no longer ex-hibit circadian rhythms of
temperature, cortisol secretion, eating, drinking, or sleep–wakefulness. Sleep
and wakefulness, for ex-ample, are taken in brief bouts throughout the 24-hour
day. Total sleep time, however, may increase under these circumstances. No
selective lesion of the human SCN has been documented.
Homeostatic Regulation of Sleep–Wakefulness
Common
experience suggests that the longer one is awake, the more likely one is to
fall asleep. Furthermore, sleep reverses the sleepiness and other consequences
of wakefulness. Thus, sleep can be said to perform a homeostatic function; it
is a time of rest and restoration that overcomes the “ravages of wakefulness”
(Daan et al., 1984). Consistent with
the hypothesis, sleep dep-rivation usually decreases sleep latency and
increases sleep ef-ficiency and delta sleep on recovery nights.
The
precise regulation of sleep and wakefulness remains an area of intense
investigation and theory. Two of the current theories of sleep–wake regulation
include the two-process model (Daan et al.,
1984) and the opponent process model (Edgar et
al., 1993). The first postulates that sleep and wakefulness are regu-lated
by a circadian process (process C), which sets the circa-dian thresholds for
sleep and wakefulness, and a homeostatic process (process S), in which sleep
propensity builds up with wakefulness and dissipates during non-REM sleep,
especially delta sleep. The opponent process model postulates that the SCN
promotes alertness and that duration of wakefulness facilitates sleep.
Related Topics