Chapter: Medical Physiology: Pituitary Hormones and Their Control by the Hypothalamus
Physiological Functions of Growth Hormone
Physiological Functions of Growth Hormone
All the major anterior pituitary hormones, except for growth hormone, exert their principal effects by stimulating target glands, including thyroid gland, adrenal cortex, ovaries, testicles, and mammary glands. The functions of each of these pituitary hormones are so intimately concerned with the functions of the respec-tive target glands that, except for growth hormone, their functions are discussed in subsequent along with the target glands. Growth hormone, in
Growth Hormone Promotes Growth of Many Body Tissues
Growth hormone, also called somatotropic hormone or somatotropin, is a small protein molecule that con-tains 191 amino acids in a single chain and has a molecular weight of 22,005. It causes growth of almost all tissues of the body that are capable of growing. It promotes increased sizes of the cells and increased mitosis, with development of greater numbers of cells and specific differentiation of certain types of cells such as bone growth cells and early muscle cells.
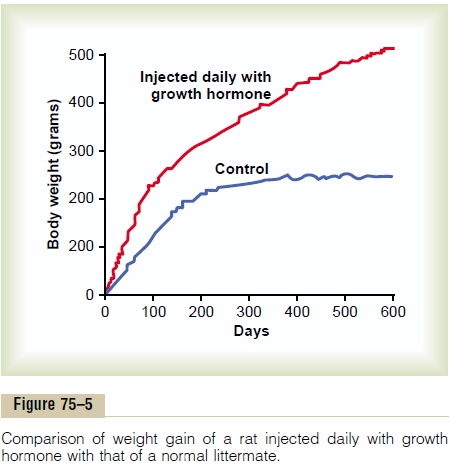
Figure 75–5 shows typical weight charts of two growing littermate rats, one of which received daily injections of growth hormone and the other of which did not receive growth hormone. This figure shows marked enhancement of growth in the rat given growth hormone—in the early days of life and even after the two rats reached adulthood. In the early stages of development, all organs of the treated rat increased proportionately in size; after adulthood was reached, most of the bones stopped lengthening, but many of the soft tissues continued to grow. This results from the fact that once the epiphyses of the long bones have united with the shafts, further lengthening of bone cannot occur, even though most other tissues of the body can continue to grow throughout life.
Growth Hormone Has Several Metabolic Effects
Aside from its general effect in causing growth, growth hormone has multiple specific metabolic effects, including (1) increased rate of protein synthesis in most cells of the body; (2) increased mobilization of fatty acids from adipose tissue, increased free fatty acids in the blood, and increased use of fatty acids for energy; and (3) decreased rate of glucose utilization throughout the body. Thus, in effect, growth hormone enhances body protein, uses up fat stores, and con-serves carbohydrates.
Growth Hormone Promotes Protein Deposition in Tissues
Although the precise mechanisms by which growth hormone increases protein deposition are not known, a series of different effects are known, all of which could lead to enhanced protein deposition.
Enhancement of Amino Acid Transport Through the Cell Mem- branes. Growth hormone directly enhances transportof at least some and perhaps most amino acids through the cell membranes to the interior of the cells. This increases the amino acid concentrations in the cells and is presumed to be at least partly responsible for the increased protein synthesis. This control of amino acid transport is similar to the effect of insulin in con-trolling glucose transport through the membrane.
Enhancement of RNA Translation to Cause Protein Synthesis by the Ribosomes. Even when the amino acid concentra-tions are not increased in the cells, growth hormone still increases RNA translation, causing protein to be synthesized in greater amounts by the ribosomes in the cytoplasm.
Increased Nuclear Transcription of DNA to Form RNA. Overmore prolonged periods (24 to 48 hours), growth hormone also stimulates the transcription of DNA in the nucleus, causing the formation of increased quan-tities of RNA. This promotes more protein synthesis and promotes growth if sufficient energy, amino acids, vitamins, and other requisites for growth are available. In the long run, this may be the most important func-tion of growth hormone.
Decreased Catabolism of Protein and Amino Acids. In addi-tion to the increase in protein synthesis, there is a decrease in the breakdown of cell protein. A probable reason for this is that growth hormone also mobilizes large quantities of free fatty acids from the adipose tissue, and these are used to supply most of the energy for the body’s cells, thus acting as a potent “protein sparer.”
Summary. Growth hormone enhances almost all facetsof amino acid uptake and protein synthesis by cells, while at the same time reducing the breakdown of proteins.
Growth Hormone Enhances Fat Utilization for Energy
Growth hormone has a specific effect in causing the release of fatty acids from adipose tissue and, therefore, increasing the concentration of fatty acids in the body fluids. In addition, in tissues throughout the body, growth hormone enhances the conversion of fatty acids to acetyl coenzyme A (acetyl-CoA) and its subsequent utilization for energy. Therefore, under the influence of growth hormone, fat is used for energy in preference to the use of carbohydrates and proteins.
Growth hormone’s ability to promote fat utilization, together with its protein anabolic effect, causes an increase in lean body mass. However, mobilization of fat by growth hormone requires several hours to occur, whereas enhancement of protein synthesis can begin in minutes under the influence of growth hormone.
“Ketogenic” Effect of Growth Hormone. Under the influ-ence of excessive amounts of growth hormone, fat mobilization from adipose tissue sometimes becomes so great that large quantities of acetoacetic acid are formed by the liver and released into the body fluids, thus causing ketosis. This excessive mobilization of fat from the adipose tissue also frequently causes a fatty liver.
Growth Hormone Decreases Carbohydrate Utilization
Growth hormone causes multiple effects that influence carbohydrate metabolism, including (1) decreased glucose uptake in tissues such as skeletal muscle and fat, (2) increased glucose production by the liver, and (3) increased insulin secretion.
Each of these changes results from growth hormone–induced “insulin resistance,” which attenu-ates insulin’s actions to stimulate the uptake and uti-lization of glucose in skeletal muscle and fat and to inhibit gluconeogenesis (glucose production) by the liver; this leads to increased blood glucose concentra-tion and a compensatory increase in insulin secretion. For these reasons, growth hormone’s effects are called diabetogenic, and excess secretion of growth hormonecan produce metabolic disturbances very similar to those found in patients with type II (non-insulin-dependent) diabetes, who are also very resistant to the metabolic effects of insulin.
We do not know the precise mechanism by which growth hormone causes insulin resistance and decreased glucose utilization by the cells. However, growth hormone–induced increases in blood concen-trations of fatty acids may impair insulin’s actions on tissue glucose utilization. Experimental studies indi-cate that raising blood levels of fatty acids above normal rapidly decreases the sensitivity of the liver and skeletal muscle to insulin’s effects on carbohy-drate metabolism.
Necessity of Insulin and Carbohydrate for the Growth-Promoting Action of Growth Hormone. Growth hormonefails to cause growth in an animal that lacks a pan-creas; it also fails to cause growth if carbohydrates are excluded from the diet. This shows that adequate insulin activity and adequate availability of carbohy-drates are necessary for growth hormone to be effec- tive. Part of this requirement for carbohydrates and insulin is to provide the energy needed for the metab-olism of growth, but there seem to be other effects as well. Especially important is insulin’s ability to enhance the transport of some amino acids into cells, in the same way that it stimulates glucose transport.
Growth Hormone Stimulates Cartilage and Bone Growth
Although growth hormone stimulates increased de-position of protein and increased growth in almost all tissues of the body, its most obvious effect is to increase growth of the skeletal frame. This results from multiple effects of growth hormone on bone, including
1. increased deposition of protein by the chondro-cytic and osteogenic cells that cause bone growth,
2. increased rate of reproduction of these cells, and (3) a specific effect of converting chondrocytes into osteogenic cells, thus causing deposition of new bone.
There are two principal mechanisms of bone growth. First, in response to growth hormone stimula-tion, the long bones grow in length at the epiphyseal cartilages, where the epiphyses at the ends of the bone are separated from the shaft. This growth first causes deposition of new cartilage, followed by its conversion into new bone, thus elongating the shaft and pushing the epiphyses farther and farther apart. At the same time, the epiphyseal cartilage itself is progressively used up, so that by late adolescence, no additional epi-physeal cartilage remains to provide for further long bone growth. At this time, bony fusion occurs between the shaft and the epiphysis at each end, so that no further lengthening of the long bone can occur.
Second, osteoblasts in the bone periosteum and in some bone cavities deposit new bone on the surfaces of older bone. Simultaneously, osteoclasts in the bone remove old bone. When the rate of deposition is greater than that of resorption, the thickness of the bone increases. Growthhormone strongly stimulates osteoblasts. Therefore, thebones can continue to become thicker throughout life under the influence of growth hormone; this is espe-cially true for the membranous bones. For instance, the jaw bones can be stimulated to grow even after ado-lescence, causing forward protrusion of the chin and lower teeth. Likewise, the bones of the skull can grow in thickness and give rise to bony protrusions over the eyes.
Growth Hormone Exerts Much of Its Effect Through Intermediate Substances Called “Somatomedins” (Also Called “Insulin-Like Growth Factors”)
When growth hormone is supplied directly to cartilage chondrocytes cultured outside the body, proliferation or enlargement of the chondrocytes usually fails to occur. Yet growth hormone injected into the intact animal does cause proliferation and growth of the same cells.
In brief, it has been found that growth hormone causes the liver (and, to a much less extent,other tissues) to form several small proteins called somatomedins that have the potent effect of increasing all aspects of bone growth. Many of the somatomedin effects on growth are similar to the effects of insulin on growth. Therefore, the somato-medins are also called insulin-like growth factors (IGFs).
At least four somatomedins have been isolated, but by far the most important of these is somatomedinC (also called IGF-I). The molecular weight ofsomatomedin C is about 7500, and its concentration in the plasma closely follows the rate of growth hormone secretion.
The pygmies of Africa have a congenital inability to synthesize significant amounts of somatomedin C. Therefore, even though their plasma concentration of growth hormone is either normal or high, they have diminished amounts of somatomedin C in the plasma; this apparently accounts for the small stature of these people. Some other dwarfs (e.g., the LĂ©vi-Lorain dwarf) also have this problem.
It has been postulated that most, if not all, of the growth effects of growth hormone result from somatomedin C and other somatomedins, rather than from direct effects of growth hormone on the bones and other peripheral tissues. Even so, experi-ments have demonstrated that injection of growth hormone directly into the epiphyseal cartilages of bones of living animals causes the specific growth of these cartilage areas, and the amount of growth hormone required for this is minute. Some aspects of the somatomedin hypothesis are still questionable. One possibility is that growth hormone can cause the formation of enough somatomedin C in the local tissue to cause local growth. It is also possible that growth hormone itself is directly responsible for increased growth in some tissues and that the somatomedin mechanism is an alternative means of increasing growth but not always a necessary one.
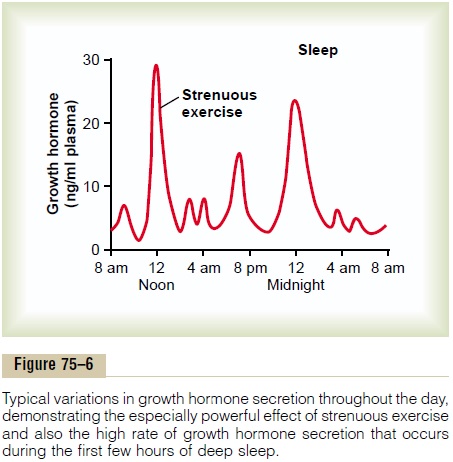
Short Duration of Action of Growth Hormone but Prolonged Action of Somatomedin C. Growth hormone attaches onlyweakly to the plasma proteins in the blood. Therefore, it is released from the blood into the tissues rapidly, having a half-time in the blood of less than 20 minutes. By contrast, somatomedin C attaches strongly to a carrier protein in the blood that, like somatomedin C, is produced in response to growth hormone. As a result, somatomedin C is released only slowly from the blood to the tissues, with a half-time of about 20 hours. This greatly prolongs the growth-promoting effects of the bursts of growth hormone secretion shown in Figure 75–6.
Regulation of Growth Hormone Secretion
For many years it was believed that growth hormone was secreted primarily during the period of growth but then disappeared from the blood at adolescence. This has proved to be untrue. After adolescence, secretion decreases slowly with aging, finally falling to about 25 per cent of the adolescent level in very old age.
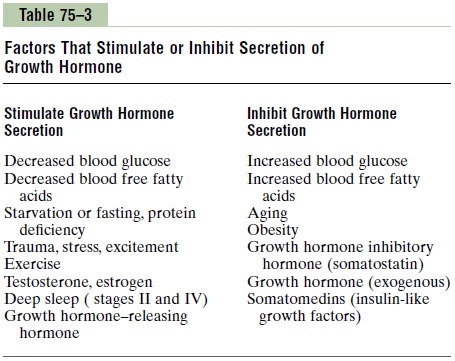
Growth hormone is secreted in a pulsatile pattern, increasing and decreasing. The precise mechanisms that control secretion of growth hormone are not fully understood, but several factors related to a person’s state of nutrition or stress are known to stimulate secretion: (1) starvation, especially with severe proteindeficiency; (2) hypoglycemia or low concentration of fatty acids in the blood; (3) exercise; (4) excitement; and (5) trauma. Growth hormone also characteristically increases during the first 2 hours of deep sleep, as shown in Figure 75–6. Table 75–3 summarizes some of the factors that are known to influence growth hormone secretion.
The normal concentration of growth hormone in the plasma of an adult is between 1.6 and 3 ng/ml; in a child or adolescent, it is about 6 ng/ml. These values often increase to as high as 50 ng/ml after depletion of the body stores of proteins or carbohydrates during prolonged starvation.
Under acute conditions, hypoglycemia is a far more potent stimulator of growth hormone secretion than is an acute decrease in protein intake. Conversely, in chronic conditions, growth hormone secretion seems to correlate more with the degree of cellular protein depletion than with the degree of glucose insufficiency. For instance, the extremely high levels of growth hormone that occur during starvation are closely related to the amount of protein depletion.
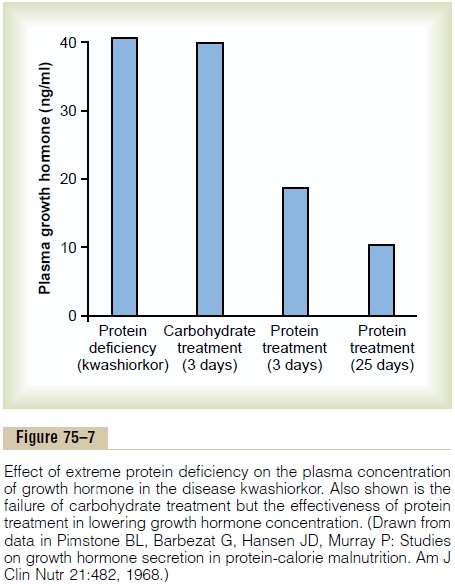
Figure 75–7 demonstrates the effect of protein defi-ciency on plasma growth hormone and then the effect of adding protein to the diet. The first column shows very high levels of growth hormone in children with extreme protein deficiency during the protein malnu-trition condition called kwashiorkor; the second column shows the levels in the same children after 3 days of treatment with more than adequate quantities of carbohydrates in their diets, demonstrating that the carbohydrates did not lower the plasma growth hormone concentration. The third and fourth columns show the levels after treatment with protein supple-ments for 3 and 25 days, respectively, with a concomi-tant decrease in the hormone.
These results demonstrate that under severe condi-tions of protein malnutrition, adequate calories alone are not sufficient to correct the excess production of growth hormone. The protein deficiency must also be corrected before the growth hormone concentration will return to normal.
Role of the Hypothalamus, Growth Hormone–Releasing Hormone, and Somatostatin in the Control of Growth Hormone Secretion
From the preceding description of the many factors that can affect growth hormone secretion, one can readily understand the perplexity of physiologists as they attempt to unravel the mysteries of regulation of growth hormone secretion. It is known that growth hormone secretion is controlled by two factors secreted in the hypothalamus and then transported to the anterior pituitary gland through the hypothalamic-hypophysial portal vessels. They are growthhormone–releasing hormone and growth hormone inhibitory hormone (also calledsomatostatin). Both ofthese are polypeptides; GHRH is composed of 44 amino acids, and somatostatin is composed of 14 amino acids.
The part of the hypothalamus that causes secretion of GHRH is the ventromedial nucleus; this is the same area of the hypothalamus that is sensitive to blood glucose concentration, causing satiety in hyper-glycemic states and hunger in hypoglycemic states. The secretion of somatostatin is controlled by other nearby areas of the hypothalamus. Therefore, it is reasonable to believe that some of the same signals that modify a person’s behavioral feeding instincts also alter the rate of growth hormone secretion.
In a similar manner, hypothalamic signals depicting emotions, stress, and trauma can all affect hypothala-mic control of growth hormone secretion. In fact, experiments have shown that catecholamines, dopamine, and serotonin, each of which is released by a different neuronal system in the hypothalamus, all increase the rate of growth hormone secretion.
Most of the control of growth hormone secretion is probably mediated through GHRH rather than through the inhibitory hormone somatostatin. GHRH stimulates growth hormone secretion by attaching to specific cell membrane receptors on the outer surfaces of the growth hormone cells in the pituitary gland. The receptors activate the adenylyl cyclase system inside the cell membrane, increasing the intracellular level of cyclic adenosine monophosphate (cAMP). This has both a short-term and a long-term effect. The short-term effect is to increase calcium ion transport into the cell; within minutes, this causes fusion of the growth hormone secretory vesicles with the cell membrane and release of the hormone into the blood. The long-term effect is to increase transcription in the nucleus by the genes to stimulate the synthesis of new growth hormone.
When growth hormone is administered directly into the blood of an animal over a period of hours, the rate of endogenous growth hormone secretion decreases. This demonstrates that growth hormone secretion is subject to typical negative feedback control, as is true for essentially all hormones. The nature of this feed-back mechanism and whether it is mediated mainly through inhibition of GHRH or enhancement of somatostatin, which inhibits growth hormone secre-tion, are uncertain.
In summary, our knowledge of the regulation of growth hormone secretion is not sufficient to describe a composite picture. Yet, because of the extreme secre-tion of growth hormone during starvation and its important long-term effect to promote protein syn-thesis and tissue growth, we can propose the follow-ing: the major long-term controller of growth hormone secretion is the long-term state of nutrition of the tissues themselves, especially their level of protein nutrition. That is, nutritional deficiency or excess tissue need for cellular proteins—for instance, after a severe bout of exercise when the muscles’ nutritional status has been taxed—in some way increases the rate of growth hormone secretion. Growth hormone, in turn, promotes synthesis of new proteins while at the same time conserving the proteins already present in the cells.
Abnormalities of Growth Hormone Secretion
Panhypopituitarism. This term means decreased secretionof all the anterior pituitary hormones. The decrease in secretion may be congenital (present from birth), or it may occur suddenly or slowly at any time during life, most often resulting from a pituitary tumor that destroys the pituitary gland.
Dwarfism. Most instances of dwarfism result from gener-alized deficiency of anterior pituitary secretion (panhy-popituitarism) during childhood. In general, all the physical parts of the body develop in appropriate pro-portion to one another, but the rate of development is greatly decreased. A child who has reached the age of 10 years may have the bodily development of a child aged 4 to 5 years, and the same person at age 20 years may have the bodily development of a child aged 7 to 10 years.
A person with panhypopituitary dwarfism does not pass through puberty and never secretes sufficient quantities of gonadotropic hormones to develop adult sexual functions. In one third of such dwarfs, however, only growth hormone is deficient; these persons do mature sexually and occasionally reproduce. In one type of dwarfism (the African pygmy and the LĂ©vi-Lorain dwarf), the rate of growth hormone secretion is normal or high, but there is a hereditary inability to form somatomedin C, which is a key step for the promotion of growth by growth hormone.
Treatment with Human Growth Hormone. Growth hor-mones from different species of animals are sufficiently different from one another that they will cause growth only in the one species or, at most, closely related species. For this reason, growth hormone prepared from lower animals (except, to some extent, from primates) is not effective in human beings. Therefore, the growth hormone of the human being is called human growthhormone to distinguish it from the others.
In the past, because growth hormone had to be pre-pared from human pituitary glands, it was difficult to obtain sufficient quantities to treat patients with growth hormone deficiency, except on an experimental basis. However, human growth hormone can now be synthe-sized by Escherichia coli bacteria as a result of success-ful application of recombinant DNA technology. Therefore, this hormone is now available in sufficient quantities for treatment purposes. Dwarfs who have pure growth hormone deficiency can be completely cured if treated early in life. Human growth hormone may prove to be beneficial in other metabolic disorders because of its widespread metabolic functions.
Panhypopituitarism in the Adult. Panhypopituitarism firstoccurring in adulthood frequently results from one of three common abnormalities. Two tumorous conditions, craniopharyngiomas or chromophobe tumors, may compress the pituitary gland until the functioning anterior pituitary cells are totally or almost totally destroyed. The third cause is thrombosis of the pituitary blood vessels. This abnormality occasionally occurs when a new mother develops circulatory shock after the birth of her baby.
The general effects of adult panhypopituitarism are (1) hypothyroidism, (2) depressed production of gluco-corticoids by the adrenal glands, and (3) suppressed secretion of the gonadotropic hormones so that sexual functions are lost. Thus, the picture is that of a lethargic person (from lack of thyroid hormones) who is gaining weight (because of lack of fat mobilization by growth, adrenocorticotropic, adrenocortical, and thyroid hor-mones) and has lost all sexual functions. Except for the abnormal sexual functions, the patient can usually be treated satisfactorily by administering adrenocortical and thyroid hormones.
Gigantism. Occasionally, the acidophilic, growthhormone–producing cells of the anterior pituitary gland become excessively active, and sometimes even aci-dophilic tumors occur in the gland. As a result, large quantities of growth hormone are produced. All body tissues grow rapidly, including the bones. If the condi-tion occurs before adolescence, before the epiphyses of the long bones have become fused with the shafts, height increases so that the person becomes a giant— up to 8 feet tall.
The giant ordinarily has hyperglycemia, and the beta cells of the islets of Langerhans in the pancreas are prone to degenerate because they become overactive owing to the hyperglycemia. Consequently, in about 10 per cent of giants, full-blown diabetes mellitus eventu-ally develops.
In most giants, panhypopituitarism eventually devel-ops if they remain untreated, because the gigantism is usually caused by a tumor of the pituitary gland that grows until the gland itself is destroyed. This eventual general deficiency of pituitary hormones usually causes death in early adulthood. However, once gigantism is diagnosed, further effects can often be blocked by microsurgical removal of the tumor or by irradiation of the pituitary gland.
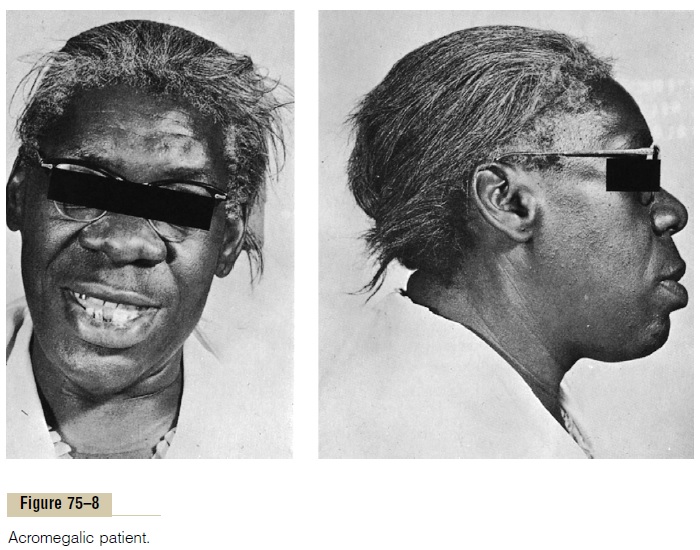
Acromegaly. If an acidophilic tumor occurs after adoles-cence—that is, after the epiphyses of the long bones have fused with the shafts—the person cannot grow taller, but the bones can become thicker and the soft tissues can continue to grow. This condition, shown in Figure 75–8, is known as acromegaly. Enlargement is especially marked in the bones of the hands and feet and in the membranous bones, including the cranium, nose, bosses on the forehead, supraorbital ridges, lower jawbone, and portions of the vertebrae, because their growth does not cease at adolescence. Consequently, the lower jaw protrudes forward, sometimes as much as half an inch, the forehead slants forward because of excess development of the supraorbital ridges, the nose increases to as much as twice normal size, the feet require size 14 or larger shoes, and the fingers become extremely thickened so that the hands are almost twice normal size. In addition to these effects, changes in the vertebrae ordinarily cause a hunched back, which is known clinically as kyphosis. Finally, many soft tissue organs, such as the tongue, the liver, and especially the kidneys, become greatly enlarged.
Possible Role of Decreased Growth Hormone Secretion in Causing Changes Associated with Aging
In people who have lost the ability to secrete growth hormone, some features of the aging process accelerate. For instance, a 50-year-old person who has been without growth hormone for many years may have the appear-ance of a person aged 65. The aged appearance seems to result mainly from decreased protein deposition in most tissues of the body and increased fat deposition in its place. The physical and physiological effects are increased wrinkling of the skin, diminished rates of function of some of the organs, and diminished muscle mass and strength.
As one ages, the average plasma concentration of growth hormone in an otherwise normal person changes approximately as follows:

Thus, it is highly possible that some of the normal aging effects result from diminished growth hormone secretion. In fact, multiple tests of growth hormone therapy in older people have demonstrated three important effects that suggest antiaging actions: (1) increased protein deposition in the body, especially in the muscles; (2) decreased fat deposits; and (3) a feeling of increased energy.
Related Topics