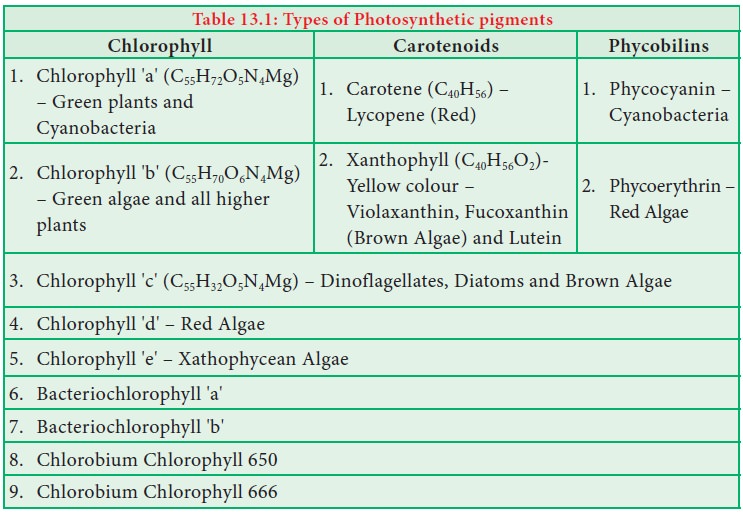
Chlorophyll, Carotenoids, Phycobilins - Photosynthetic Pigments┬Ł | 11th Botany : Chapter 13 : Photosynthesis
Chapter: 11th Botany : Chapter 13 : Photosynthesis
Photosynthetic Pigments┬Ł
Photosynthetic Pigments┬Ł
A
photosynthetic pigment is a pigment that is present in chloroplasts or photosynthetic bacteria which captures the
light energy necessary for photosynthesis (Table 13.1).
1. Chlorophyll
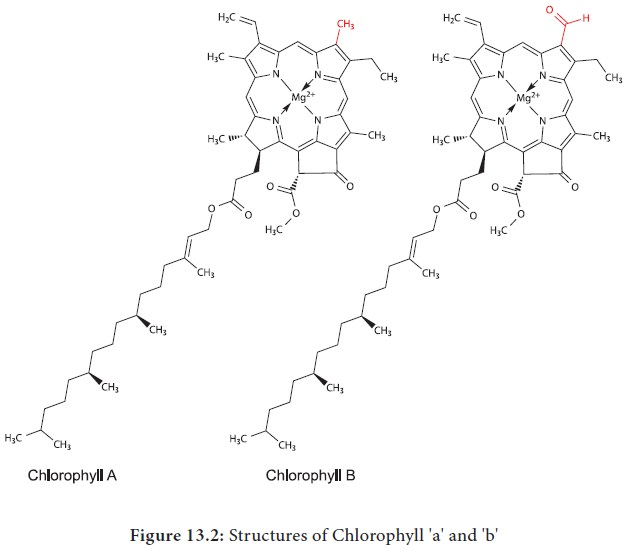
Chlorophyll a is the primary pigment which acts as a reaction centre and all other pigments act as accessory pigments and trap solar energy and then transfer it to chlorophyll 'a'. Chlorophyll molecules have a tadpole like structure.
It
consists of Mg- Porphyrin head (Hydrophilic Head) and (Lipophilic tail) Phytol
tail. The Porphyrin head consists of four pyrrol rings linked together by C-H
bridges. Each pyrrole ring comprises of four carbons and one nitrogen atom.
Porphyrin ring has several side groups which alter the properties of the
pigment. Different side groups are indicative of various types of chlorophyll.
The Phytol tail made up of 20 carbon alcohol is attached to carbon 7 of the
Pyrrole ring IV. It has a long propionic acid ester bond. Long lipophilic tail
helps in anchoring chlorophyll to the lamellae (Figure 13.2).
i. Biosynthesis of Chlorophyll
Chlorophyll
is synthesized from intermediates of respiration and
Succinic acid an intermediate of Krebs cycle is activated by the addition of
coenzyme A and it reacts with a simple amino acid glycine and the reaction goes
on to produce chlorophyll 'a'. Bio synthesis of chlorophyll a requires Mg,
Fe, Cu, Zn, Mn, K and nitrogen. The absence of any one of these minerals leads
to chlorosis (Recall what you have studied in ŌĆśMineral NutritionŌĆÖ).
ii. Comparison of Chlorophyll ŌĆō a with other pigments
1. Chlorophyll 'b' differs from Chlorophyll 'a' in having CHO (aldehyde)group instead of CH3(Methyl) group at the 3rd C atom in II Pyrrol ring (Figure 13.2).
2. Chlorophyll 'c' differs from Chlorophyll 'a' by lacking phytol tail.
3. Chlorophyll 'd' differs from Chlorophyll 'a' in having O-CHO group instead of CH-CH2 group at 2nd Carbon in the 1st Pyrrol ring.
4.
Pheophytin resembles Chlorophyll 'a' except that it
lacks Mg atom. Instead it has two H atoms.
5.
Phycobilins have open tetra pyrrols and they have
neither Mg nor phytol chain.
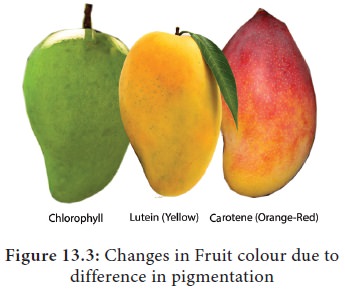
2. Carotenoids
Carotenoids
are yellow to orange pigments, mostly tetraterpens and these pigments absorb
light strongly in the blue to violet region of visible spectrum. These pigments
protect chlorophyll from photo-oxidative damage. Hence, they are called as shield pigments. These pigments absorb light and transfer these to
chlorophyll. Almost all carotenoid pigments have 40 carbon atoms. Ripening of
fruits, floral colours and leaf colour change during autumn is due to
Carotenoids (Carotene and Xanthophyll) (Figure 13.3).
i. Carotenes:
Orange, Red, Yellow and Brownish pigments, hydrocarbons (Lipids) and most of them are tetraterpenes(C40H56). Carotene is the most abundant Carotene in plants and it is a precursor of Vitamin ŌĆēA. Lycopene is the red pigment found in the fruits of tomato, red peppers and roses.
ii. Xanthophylls:
Yellow (C40H56O2)
pigments are like carotenes but contain oxygen. Lutein is responsible for
yellow colour change of leaves during autumn season. Examples: Lutein, Violaxanthin
and Fucoxanthin.
3. Phycobilins
They are
proteinaceous pigments, soluble in water, and do not contain Mg and Phytol
tail. They exist in two forms such as 1. Phycocyanin found in cyanobacteria 2.
Phycoerythrin found in rhodophycean algae (Red algae).
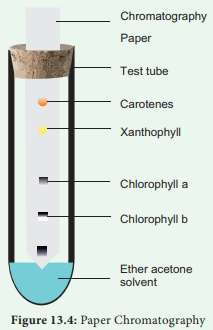
Separation of Chloroplast pigments by paper Chromatography method
Step 1. Extract chlorophyll pigment from the leaves using 80% Acetone.
Step 2. Allow to concentrate by evaporation.
Step 3. Apply few drops on one end above 2 cm from the edge of a chromatographic paper.
Step 4. A solvent with mixture of Petroleum ether and acetone in the ratio of 9:1 is prepared and poured into development chamber.
Step 5. Place the strip above the solvent by placing one end of the strip touching the solvent.
Observation
After one hour observe the chromatographic paper. You can find the pigments being separated into four distinct spots (Figure 13. 4).
Related Topics