Chapter: 11th Botany : Chapter 13 : Photosynthesis
Chemiosmotic Theory
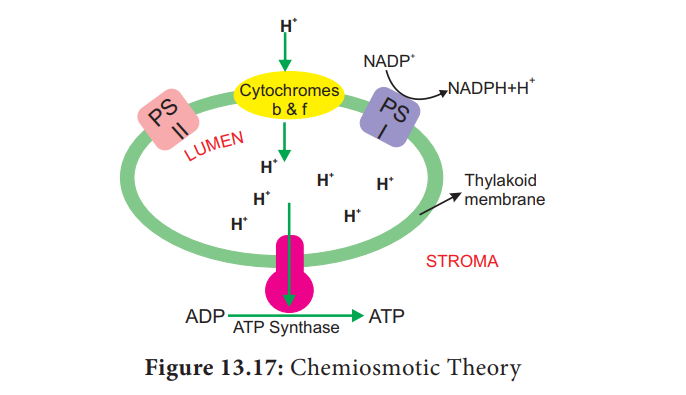
Chemiosmotic Theory
Chemiosmosis
theory was proposed by P. Mitchell (1966).
According to this theory electrons
are transported along the membrane through PS I and PS II and connected by
Cytochrome b6-f complex. The flow of electrical current is due to difference in
electrochemical potential of protons across the membrane. Splitting of water
molecule takes place inside the membrane. Protons or H+ ions
accumulate within the lumen of the thylakoid (H+ increase 1000 to
2000 times). As a result, proton concentration is increased inside the
thylakoid lumen. These protons move across the membrane because the primary
acceptor of electron is located outside the membrane. Protons in stroma less in
number and creates a proton gradient. This gradient is broken down due to the
movement of proton across the membrane to the stroma through CFO of
the ATP synthase enzyme. The proton motive force created inside the lumen of
thylakoid or chemical gradient of H+ ion across the
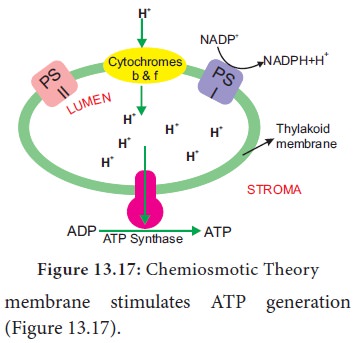
The
evolution of one oxygen molecule (4 electrons required) requires 8 quanta of
light. C3 plants utilise 3 ATPs and 2 NADPH + H+ to
evolve one Oxygen molecule. To evolve 6 molecules of Oxygen 18 ATPs and 12
NADPH + H+ are utilised. C 4 plants utilise 5 ATPs and 2
NADPH + H+ to evolve one oxygen molecule. To evolve 6 molecules of
Oxygen 30 ATPs and 12 NADPH + H+ are utilised.
Check your grasp!
What will be the quanta requirement
for complete light reaction which releases 6 oxygen molecules?
Solution: Complete light reaction
releases 6 oxygen molecules. If one molecule of oxygen evolution requires 8
quanta means, for 6 oxygen molecules 6 Ă— 8 = 48 quanta of light required for
complete light reaction.
Related Topics