Chapter: Modern Medical Toxicology: Hydrocarbons and Pesticides: Pesticides
Paraquat and Diquat - Herbicides (Weedicides)
HERBICIDES (WEEDICIDES)
These
are compounds which kill weeds. Examples acrolein, dalaphon, paraquat, diquat,
glyphosate, atrazine, propazine, simazine, nitrofen, trichloroacetic acid, and
chlorophenoxy compounds.
Paraquat and Diquat
Paraquat and diquat are widely used
herbicides which belong to the bipyridyl group.* Paraquat is
1,1-dimethyl-4,4-bipyridy-lium dichloride, and was first synthesized in 1882,
but began to be used as a herbicide only since the 1960s. It is available
either in granular form (25–80 gm/kg) or as water soluble concen-trate which is
an odourless brown liquid (100–200 gm/L). The granular form is available as
colourless crystals (dichloride salt) or a yellow solid (bis(methyl sulfate)
salt). In India, most of the concentrates of paraquat are available as 10–20%
solu-tions, and therefore 10 ml of a 20% solution can contain about 2 grams of
paraquat. Common brand names include Weedol,
Gramoxone and Uniquat.
Diquat is 1,1-
ethylene-2,2-dipyridylium dibromide, and is less commonly used than paraquat.
It has the same indica-tions and mode of action as paraquat but produces much
less severe pulmonary lesions.
Toxicokinetics
Absorption
through inhalation, skin contact, or eye contact is minimal, though prolonged
contact can be hazardous. On ingestion, paraquat solution is much more rapidly
absorbed than the granular form. After absorption it tends to accumu-late in
the lungs and kidneys. Paraquat has a large volume of distribution (1.2 to 1.6
L/kg). More than 90% of an absorbed dose is excreted by the kidneys as the
parent compound within 12 to 24 hours. Paraquat is distributed into all organs.
Highest concentrations are found in kidney and lung; paraquat also accumulates
in muscle tissue, which may represent a reservoir, explaining prolonged
detection of plasma or urine paraquat weeks or months following ingestion.
Mode of Action
■■ Paraquat is a
rapidly-acting herbicide. It kills the tissues of green plants by contact action with foliage and by
some amount of translocation to the xylem.
■■Corneal injury and protracted
opacification of the cornea may result following eye exposure to paraquat.
Extensive loss of superficial areas of the corneal and conjunctival ![]() epithelium may occur. Healing,
although slow, is usually complete if given prompt medical care.
epithelium may occur. Healing,
although slow, is usually complete if given prompt medical care.
■■ Irritation
of the skin and mucous membranes may be severe following paraquat exposure.
■■ After
ingestion, sore throat and difficulty in swallowing can occur. Irritation of
the gut including abdominal pain, nausea, vomiting, and diarrhoea may occur
immediately following ingestion. Concentrated solutions of paraquat corrode the
GI mucosa. Tachycardia, hypotension, and cardiorespiratory arrest can occur
with large ingestions. Cerebral oedema may occur. Pancreatitis may develop in
some cases of acute paraquat poisoning, and can cause severe abdominal pain.
■■ The
maximum damage is seen in the lungs where cellular injury is initiated by the
NADPH-dependant reduction of paraquat to the monocation radical (PQ+). Reaction
with molecular oxygen yields the superoxide radical (O2-) and
reforms the paraquat dication, ready to be reduced again. This process known as
redox cycling is sustained by the extensive supply of electrons and oxygen in
the lungs. This and the subsequent reactions explain why oxygen enhances the
toxicity of paraquat, and paraquat enhances the toxicity of oxygen. Two superoxide
species form hydrogen peroxide in a reaction catalysed by superoxide dismutase.
Superoxide and hydrogen peroxide undergo a series of iron-catalysed reactions
to yield the hydroxyl radical (OH) which is thought to be the ultimate toxic
element. The hydroxyl radical causes degradation of cell membranes through
lipid peroxidation resulting in cellular death.
Usual Fatal Dose
Estimated
lethal dose is 10 to 15 ml of the concentrate. Ingestion of 20 to 40 mg of
paraquat ion per kg body weight (7.5–15.0 ml of 20% (w/v) paraquat concentrate)
results in death in most cases. Prudence requires that all cases of paraquat
ingestion be treated as potentially fatal poisonings.
Clinical Features
·
Typical
Form: (ingestion of 30 to 50
mg/kg of paraquat)
o
Initial Phase—pain in the mouth,
oesophagus, and stomach due to corrosion, vomiting, diarrhoea, dysphagia,
aphonia. There may be gastric perforation/ GI haemorrhage.
o
Second Phase—begins after 2 to 5
days and is char-acterised by renal and hepatic toxicity, i.e. renal tubu-lopathy
and centrilobular hepatic necrosis respectively. Although hepatic injury from
exposure to paraquat may be quite severe, clinical outcome is generally not
determined by hepatotoxic effects.
o
Third Phase—begins after 5 days and
is characterised by pulmonary fibrosis which leads to progressive respiratory
failure.
·
Hyperacute Form: (ingestion
of more than 50 mg/kg ofparaquat)
There is rapid development of
cardiogenic shock ending in death in 3 to 4 days. Renal and hepatic lesions are
also common.
·
Subacute Form: (ingestion
of less than 30 mg/kg ofparaquat) This is characterised only by
gastrointestinal manifesta-tions.
Mortality in paraquat poisoning can
be high and is related to two factors—concentration and quantity. Ingestion of
20% solution is associated with high mortality. Swallowing more than a mouthful
can cause death in 72 hours because it corre-sponds to ingestion of more than
50 mg/kg. If it is less than a mouthful, death may be delayed upto 70 days and
is usually due to pulmonary fibrosis. Pneumothorax, pneumopericardium and
subcutaneous emphysema may develop in patients with paraquat induced lung
injury.
Survivors of severe paraquat
poisoning often develop progressive pulmonary fibrosis within 5 to 10 days or
longer after exposure. Continued survival is dependant on the extent of lung
involvement.
Occupational exposure to paraquat
can cause a dry, cracking dermatitis and nail atrophy.
Diagnosis
·
X-ray of the chest may reveal patchy
infiltration in the early stages, and opacification of one or both lung fields
in later stages. However, if death is due to the hyperacute form of
presentation, no abnormalities may be noted on the chest X-ray.
·
Plasma paraquat level can be assayed
by spectroscopy, radioimmunoassay, or HPLC. Serum levels greater than 0.2 mcg/ml
at 24 hours, and 0.1mcg/ml at 48 hours are associated with high mortality.
·
Urine can be tested for gross
amounts of paraquat by alka-lising 3 to 5 ml with a few mg of sodium
bicarbonate, then adding a few mg of sodium dithionite. An intense blue-green
colour is a positive test.
·
Urine paraquat level can be assayed
by spectrophotometry. Survival is usually associated with levels less than
1mcg/ ml, while mortality is high when the level exceeds 10 mcg/ ml.
·
When submitting samples for chemical
analysis it must be ensured that only plastic containers are used, since
paraquat binds to glass.
·
Monitor renal and liver function
tests carefully. Obtain baseline urinalysis and monitor urine output.
·
Obtain baseline pulmonary function
tests, chest X-ray, and ABGs and monitor serially for several days.
Treatment
·
All cases of paraquat ingestions should be considered as
medical emergencies even if the patient is asymptomatic.
·
Perform upper gastrointestinal endoscopy to identify the
extent and severity of corrosion.
·
Stomach wash may be beneficial only if done within 1 hour of
ingestion. Emesis and cathartics are contraindicated. Activated charcoal is of
doubtful value.
·
Pain due to corrosion may be relieved by ice-cold fluids
(e.g. ice cream), mouthwashes, local anaesthetic sprays, and lozenges. Opiates
may be required in some cases.
·
Haemodialysis or haemoperfusion may be beneficial if
undertaken within the first 10 to 12 hours.
·
Supportive measures form the mainstay of treatment :
protection of airway, maintenance of circulation, treatment of secondary
infection, prevention or treament of renal failure, and treatment of
complications. Oxygen mustnot be
administered as far as possible since it enhances lung damage. Allow
additional oxygen only in victimsconsidered beyond rescue to relieve air hunger
and terminal disease.
·
N-acetylcysteine may be of value. There are indications that
if intravenous n-acetylcysteine and early haemodialysis (within 4 hours of
ingestion) are undertaken, survival rate may improve.
·
The combination of corticosteroids and cyclophospha-mide has
shown promise in reducing paraquat mortality, although efficacy has not been
proven in prospective controlled clinical trials. In one prospective,
randomised study, patients received gastric lavage followed by activated
charcoal instillation, two 8-hour haemoper-fusion sessions against activated
charcoal, and 10 mg intravenous dexamethasone every 8 hours for 14 days. The
patients randomised into the treatment group also received at the end of
haemoperfusion 1 gram of intra-venous methylprednisolone daily for days 1, 2,
and 3, and cyclophosphamide 15 mg/kg daily for days 2 and 3 of pulse therapy.
In a single case reported separately, recovery was achieved in a severely
poisoned paraquat patient by a second pulse of methylprednisolone on day 30
when pulmonary inflammation and hypoxaemia emerged despite steady daily therapy
of dexamethasone after the first pulse therapy. More study of a larger number
of severely poisoned patients must be performed to confirm or refute benefit of
this approach before it can be recommended as a standard treatment.
·
Non-steroidal anti-inflammatory agents, colchicine, collagen
synthesis inhibitors, desferrioxamine, or total exclusion from external
respiration may prevent lung fibrosis. However, the efficacy of these
treatments has yet to be established in the treatment of human paraquat
poisonings.
·
Pulmonary damage may be ameliorated by radiotherapy. However
the current consensus is NOT to undertake radio-therapy because of lack of
clinical evidence of efficacy.
Lung transplantation has not met
with success in most cases where it was attempted, though some recent reports
indicate that it could be beneficial. Nitric oxide inhalation to maintain
tissue oxygenation in anticipation of lung trans-plantation once all absorbed
paraquat has been eliminated, is recommended by some investigators.
Autopsy Features
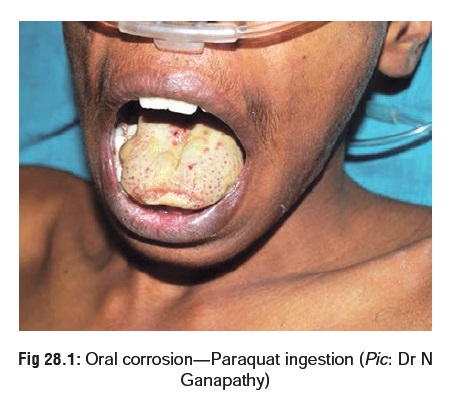
· Ulceration around lips and mouth, reddened or desquamated oral and oesophageal mucosa (Fig 28.1), erosion and patchy haemorrhages in the stomach.
·
Liver may show pallor or mottled
fatty change; centri-lobular necrosis.
·
Lungs often appear stiffened. There
may be evidence of proliferative pulmonary fibrosis, fibrinous pleurisy, or
scanty blood-stained pleural effusion. Cut surface reveals oedema.
·
Kidneys may reveal evidence of
tubular damage.
Related Topics