Chapter: Modern Medical Toxicology: Miscellaneous Drugs and Poisons: Anti-Infectives
Other Antibacterial Drugs
Other Antibacterial Drugs (in
random order)
Clindamycin
Clindamycin is a congener of
lincomycin, and though it is not structurally related to erythromycin and
chloramphenicol, it has a similar mode of action, i.e. binding to the 50s
subunit of bacterial ribosomes and suppression of protein synthesis. It is
administered mainly for anaerobic infections either orally or parenterally. It
is especially effective in infections due to Bacteroides fragilis, and in some staphylococcal and strepto-coccal
infections. Clindamycin has antiprotozoal actions also, and is administered
systemically in combination with other antiprotozoal agents for the treatment
of babesiosis, malaria, and toxoplasmosis. Clindamycin is also used for the
topical treatment of acne vulgaris as a gel or cream.
Adverse effects include diarrhoea
which may sometimes be due to a serious complication—pseudomembranous colitis.
This is in fact a widely reported adverse effect of lincomycin and clindamycin
therapy when administered orally ![]() and/or parenterally. The colitis
usually presents with loose stools or diarrhoea that may be bloody. In cases of
more severe disease, patients may have fever, leucocytosis, nausea and
vomiting, tenesmus, and abdominal tenderness or cramping. Diagnosis can be
established by proctoscopy, sigmoidoscopy, or by a barium contrast study. If
pseudomembranous colitis is present, these procedures may reveal erythematous,
friable mucosa covered with small, raised, yellowish-white plaques, and the
formation of pseudomembranes. Symptoms usually appear after 5 to 10 days of
antibiotic therapy, although this can be variable. Protein-losing enteropathy,
toxic megacolon, and perforation of the colon occur occasionally, and are
serious complications that may lead to shock and death. Even topical
clindamycin, used to treat facial acne, has caused pseudomem-branous colitis.
and/or parenterally. The colitis
usually presents with loose stools or diarrhoea that may be bloody. In cases of
more severe disease, patients may have fever, leucocytosis, nausea and
vomiting, tenesmus, and abdominal tenderness or cramping. Diagnosis can be
established by proctoscopy, sigmoidoscopy, or by a barium contrast study. If
pseudomembranous colitis is present, these procedures may reveal erythematous,
friable mucosa covered with small, raised, yellowish-white plaques, and the
formation of pseudomembranes. Symptoms usually appear after 5 to 10 days of
antibiotic therapy, although this can be variable. Protein-losing enteropathy,
toxic megacolon, and perforation of the colon occur occasionally, and are
serious complications that may lead to shock and death. Even topical
clindamycin, used to treat facial acne, has caused pseudomem-branous colitis.
The symptoms of non-specific colitis
are very similar to pseudomembranous colitis including bloody diarrhoea,
tenesmus, abdominal pain, and fever. The difference with non-specific colitis
is that there are no pseudomembranes present during proctoscopic examinations,
and rectal biopsies show non-specific inflammatory changes.
Cardiac arrhythmias, dermatitis,
nephrotoxicity, hepato-toxicity, skin rashes, erythema multiforme, anaphylaxis,
and haematological abnormalities have also been reported with clindamycin.
Rapid administration of large doses has resulted in ventricular arrhythmias,
hypotension and cardiac arrest.
Lincomycin and clindamycin may
augment pancuronium-induced neuromuscular blockade as well as produce
neuromus-cular blockade when administered alone.
Clindamycin has been reported to
cause hepatotoxicity, including elevated liver enzyme levels and cholestatic
liver disease with reduced numbers of bile ducts. Acute renal failure has
occurred following combination therapy of gentamicin and clindamycin.
Acute ingestion of lincomycin and
clindamycin has not been associated with significant toxicity. Gastrointestinal
decontamination is generally NOT necessary. The use of diphe-noxylate
hydrochloride-atropine sulfate is NOT recommended for the treatment of
lincomycin- or clindamycin-induced diar-rhoea. Discontinuation of the drug
usually results in improve-ment of the diarrhoea.
For pseudomembranous colitis: Metronidazole 750 mg orallyevery 6
hours for 7 days can rapidly eliminate C.
difficile toxin from the stools and shorten the course of illnessis. Vancomycin
therapy, 500 mg orally every 6 hours for 7 to 10 days is an alternative
therapy. In debilitated patients with recurrent C.difficile colitis, parenteral gamma-globulin (400 mg/kg
intra-venously every 3 weeks), and replacement of colonic bacteria with Saccharomyces boulardii, a
non-pathogenic yeast, have been advocated.
Neither haemodialysis nor peritoneal
dialysis appear to be effective in reducing lincomycin or clindamycin levels
significantly.
Vancomycin
Vancomycin is a chromatographically
purified, tricyclic glycopeptide antibiotic derived from Amycolatopsis orientalis (formerly Nocardia orientalis). It is very useful in the treatment of serious
infections caused by methicillin-resistant staphylo-cocci and
penicillin-resistant pneumococcal infections. It is also effective against Clostridium difficile which causes
pseudomem-branous colitis. Vancomycin is usually only administered
intravenously. It may be administered orally for treatment of staphylococcal
enterocolitis and antibiotic-associ-ated pseudomembranous colitis caused by C. difficile. Parenteral administration
is not effective for the above indications.
Adverse effects include skin rashes,
anaphylaxis, fever, ototoxicity (sensorineural hearing loss), and
nephrotoxicity. The actual number of cases of ototoxicity associated with
vancomycin use is small, and most cases of permanent hearing loss have been
associated with the co-administration of an aminoglycoside. Severe lacrimation
and conjunctivitis have been seen with therapeutic use. Nephrotoxicity can
occur with excessive serum levels but is usually reversible upon
discon-tinuation of the drug. Reversible neutropenia which usually develops
within the first week or more after the start of therapy or after a total dose
of 25 grams or more has been reported in several dozen individuals. The effects
promptly reverse when therapy is withdrawn.
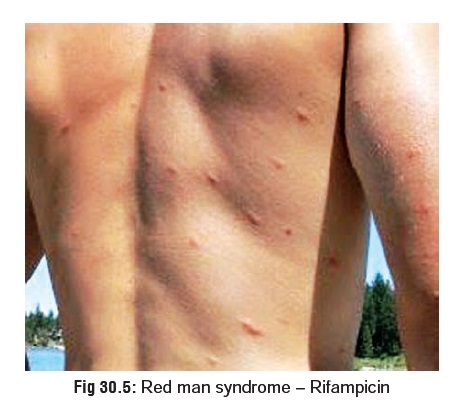
Rapid IV infusion can cause
tachycardia and hypotension. Occasionally, this results in the Red man syndrome (Fig 30.5)* or Red neck syndrome, which is characterised
by hypotension, pruritis, cutaneous flushing (face, neck, chest, and arms),
chest pain, and dyspnoea. Earlier it was thought to be caused by impurities in
the drug formulation, but now it is postulated that the syndrome occurs because
of vancomycin-induced release of endogenous histamine. Reactions generally
resolve within 20 minutes, but may persist for several hours. The incidence and
severity can be minimised by antihistamine prophylaxis, lower and more frequent
vancomycin dosing, and 2-hour infusions.
Acute vancomycin overdose results in
oliguria and renal failure. Toxicity is reported at levels sustained above 80
to 100 mcg/ml. Hypotension, apnoea, deafness, and flushed skin have been
reported after overdose. Treatment involves multi-dose activated charcoal (even
if vancomycin has been given IV), and institution of supportive measures.
Gastrointestinal absorption of vancomycin is negligible; decontamination is
rarely indicated unless coingestants are involved. Monitor the patient for
development of possible ototoxicity, nephrotoxicity, haematopoietic, or cardiac
abnormalities. While haemodialysis and charcoal haemoperfusion are not
effective, continuous arteriovenous haemofiltration may be beneficial. However
there are some reports of efficacy with haemodialysis also.
Teicoplanin
Teicoplanin obtained from Actinoplanes teichomyetius is a new antibiotic with a spectrum of activity similar to vancomycin.
However,
unlike the latter, it can be given by intramuscular injection. Once-daily
dosing is sufficient for the treatment of most infections since it has a
prolonged serum elimination half-life. Adverse effects include skin rash,
fever, neutropenia, and ototoxicity (rare).
Spectinomycin
It
is an antibiotic produced by Strep.
spectabilis, and is used mainly for the treatment of gonorrhoea in patients
who are intolerant or allergic to beta-lactam antibiotics and quinolones.
Spectinomycin is given by intramuscular injection, and in rare instances can
cause urticaria, fever, chills, vertigo and insomnia.
Polymyxin B and Colistin (Polymyxin E)
These
antibiotics obtained from Bacillus
species of micro-organisms are highly nephrotoxic and hence not advised to be
administered systemically. Polymyxin B sulfate is available in India for
ophthalmic, otic, and topical use, as well as for systemic use.
Bacitracin
It
is an antibiotic produced by Bacillus
subtilis, and is actually a group of polypeptides, the most active of which
is Bacitracin A. It is mainly employed for ophthalmic and topical use in
combination with other drugs such as neomycin, polymyxin, and hydrocortisone.
The major use of bacitracin is topical treatment of gram-positive infections on
the skin or in the eye. The drug is also used prophylactically to prevent
dermal infec-tions. Intramuscular bacitracin is available to treat infants with
pneumonia and empyema caused by susceptible staphylococci; however, it is
rarely used because of the availability of more effective and less toxic
agents. Parenteral use is associated with serious nephrotoxicity and
ototoxicity. Transient epigastric distress, including nausea, vomiting,
diarrhoea, or anal itching or burning may occur with therapeutic use. Several
cases of anaphylaxis have been reported with the use of bacitracin ointment.
Related Topics