Chapter: Modern Medical Toxicology: Miscellaneous Drugs and Poisons: Anti-Infectives
Antibacterials - Antimicrobials
ANTIMICROBIALS
Antibacterials
Sulfonamides
Sulfonamide
drugs are derivatives of para-aminobenzenesul-fonic acid. The sulfonamides were
the first ever drugs used systemically for treatment of bacterial infections.
Prontosil was the earliest such compound proved to have chemotherapeutic value,
the discovery of which in 1935 led
to the Nobel prize being awarded (in 1938)
to Domagk, a German scientist. This
was followed by the development of a profusion of similar compounds which were
used extensively in therapeutics till the advent of penicillin in the 1940s.
Examples
Sulfadiazine,
sulfacetamide, sulfamethoxazole, sulfanilamide, sulfisoxazole, sulfadoxine,
sulfasalazine, sulfacytine, silver sulfadiazine, sulfaguanidine, sulfamethizole, sulfapyridine,
sulfisoxazole and mafenide.
Today the only systemic sulfonamide
that is still popular as an antibacterial is sulfamethoxazole which is usually
combined with trimethoprim (for synergistic effect) in a ratio of 5:1, the
combined product being referred to as co-trimoxazole.
![]() Sulfacetamide is used as a topical
preparation, as also silver sulfadiazine and mafenide.
Sulfacetamide is used as a topical
preparation, as also silver sulfadiazine and mafenide.
Sulfadoxine is a long-acting
sulfonamide which is usually used in combination with pyrimethamine in the
prophylaxis and treatment of malaria.
Sulfasalazine is a poorly absorbed
sulfonamide which is used in the therapy of ulcerative colitis.
Uses
Micro-organisms
susceptible to sulfonamides include Streptococcus
pyogenes, Strep. pneumoniae, Haemophilus influ enzae, H.ducreyi, Nocardia,
Actinomyces, Calymmatobacterium granulomatis, and Chlamydia trachomatis.
Toxicokinetics
Except
for locally acting preparations, all sulfonamides are rapidly absorbed from the
GI tract. Peak plasma levels are achieved in 2 to 6 hours. Binding to plasma
proteins (especially albumin) is notable, though variable, depending on the
exact compound. Sulfonamides are distributed throughout all tissues and body
fluids, and readily pass through placenta. Metabolism occurs by acetylation in
the liver, and excretion is mainly through the urine (upto 20% as unchanged
parent compound).
Mode of Action
·
Sulfonamides are bacteriostatic in
normal doses and bacte- riocidal in extremely high concentrations.
·
They act therapeutically by
inhibiting para-aminobenzoic acid or para-aminoglutamic acid required for the
biosyn-thesis of folic acid.
Adverse Effects
·
Crystalluria: This is particularly common with
oldersulfonamides (such as sulfadiazine) which are insoluble and get
precipitated in acid urine, producing crystalline deposits that can cause
urinary obstruction. The risk can be minimised by ensuring a minimum daily
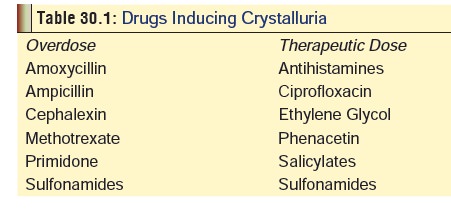
urine flow of 1200 ml (in adults), and alkalinisation. Table 30.1 provides a list of agents that can cause crystalluria.
·
Acute haemolytic anaemia, aplastic anaemia, and
agranu-locytosis.
·
Hypersensitivity reactions are not infrequent and may take
the form of dermal or mucous membrane manifestations such as skin rash,
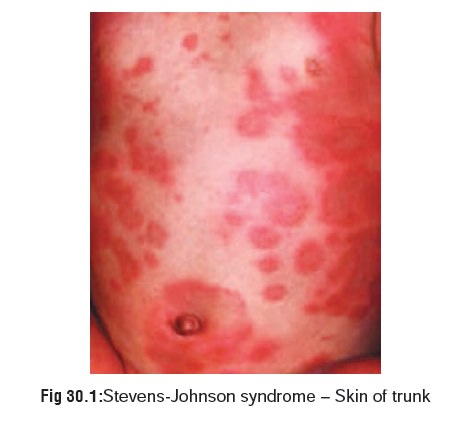
erythema nodosum, Stevens-Johnson syndrome (Fig 30.1),* Behcet’s syndrome,** and exfolia-tive dermatitis.
Stevens-Johnson and Lyell’s Syndromes are usually associated with the use of a
long-acting sulfona-mide, although other sulfonamides have been reported to
cause these reactions. This serious reaction has been reported even with the
use of ophthalmic preparations. Transient myopia, conjunctivitis, and keratitis
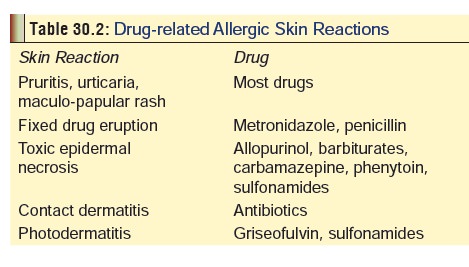
may occur in association with hypersensitivity reaction. Table 30.2 lists some common drug-related causes of skin allergy.
·
Headache, depression, and hallucinations have been reported
with therapeutic use of sulfonamides. Tremor occurred in one patient following
a fixed-dose combination of trimethoprim/sulfamethoxazole.
·
Hepatocellular, cholestatic, or mixed types of hepatitis
have been reported with therapeutic doses of sulfonamides.
·
Administration of sulfonamides to premature infants leads to
kernicterus.
·
A higher rate of adverse reactions are reported with AIDS
patients who receive sulfonamides, which may be because of increased
sensitivity to reactive sulfonamide metabolites.
Clinical (Toxic) Features
Cases of overdose involving
sulfonamides have rarely been reported. Toxicity is associated with nausea,
vomiting, diarrhoea, facial swelling headache, mental confusion, convul-sions,
and bone marrow depression. Methaemoglobinaemia can occur. Renal failure has
been reported with trimethoprim. Coma and seizures were reported following a
large overdose of sulfasalazine in one patient.
Diagnosis
The
hepatotoxicity and nephrotoxicity associated with these pharmaceuticals may
alter lab tests of liver function and kidney function including alkaline
phosphatase, bilirubin, transami- nase, cephalin flocculation, BUN, creatinine,
urine protein, etc. Monitor the haematopoietic system in long-term treatment,
even at normal doses.
·
Bedside
test: Place 1 drop of patient’s urine on wood pulp (lignin) or
pulp paper (newspaper), and add to it 1 drop ofconcentrate HCl. Normal urine
stains yellow, sulfa deriva-tives stain orange. The test can also be done on
pulverisedsulfa tablet directly.
·
Quantitation in serum can be done
with HPLC.
Treatment
·
Stomach wash.
·
Haematologic evaluation.
·
Diazepam for convulsions.
·
Determine the methaemoglobin
concentration, and evaluate the patient for clinical effects of
methaemoglobinaemia (dyspnoea, headache, fatigue, CNS depression,
tachycardia,acidosis, etc.). Treat patients with symptomatic methaemo- methaemoglobin
levels above 20 to 30%, but may occur at lower methaemoglobin levels in
patients with anaemia, or underlying pulmonary or cardiovascular disorders). Dose: 1 to 2 mg/kg/dose (0.1 to 0.2
ml/kg/dose) IV over 5 minutes, as needed every 4 hours.
·
If kidney function is normal,
administer 0.45% sodium chloride in D5W, and a diuretic such as furosemide 1
mg/ kg to a maximum of 40 mg/dose to obtain a urine flow of 3 to 6 ml/kg/hr to
increase renal excretion. For anuria or agranulocytosis, dialysis and/or
isolation should be consid-ered.
·
Supportive measures.
·
For
acute allergic reaction: The drug should be immedi-ately
discontinued and the patient observed for the possi-bility of anaphylactic
shock. In this situation the normaltreatment for anaphylaxis is carried out
with the establish-ment of an open airway, adrenaline, and diphenhydramine.
·
Intravenous N-acetylcysteine (24 g
over 3 days) was reported to be effective in treating hepatitis cause
bysulfasalazine in one reported case.
·
Haemodialysis may be beneficial.
Related Topics