Chapter: Modern Medical Toxicology: Hydrocarbons and Pesticides: Pesticides
Organophosphates (Organophosphorus Compounds) - Insecticides
INSECTICIDES
These are compounds which kill or
repel insects and related species. For example, organophosphates, carbamates,
organo-chlorines, pyrethrum and its derivatives (pyrethroids).
Organophosphates (Organophosphorus Compounds)
It
is true that calling these compounds “organophosphates” is not correct, and
they should be referred to as “organophos-phorus compounds”. But,
“organophosphates” is such an irre-sistibly compact expression.
Organophosphates
are among the most popular and most widely used insecticides in India. Table 28.1 lists common varieties along
with respective brand names.



Physical Appearance
These
compounds are available as dusts, granules, or liquids. Some products need to
be diluted with water before use, and some are burnt to make smoke that kills
insects.
Usual Fatal Dose
Toxicity
Rating*:
The following compounds are
extremely toxic (LD50: 1 to 50 mg/kg),
or highly toxic (LD50: 51 to 500 mg/kg)—
Chlorfenvinphos, Chlorpyriphos,
Demeton, Diazinon, Dichlorvos, Dimethoate, Disulfoton, Ediphenphos, Ethion,
Fenitrothion, Fensulfothion, Fenthion, Fonophos, Formothion, Methyl Parathion,
Mevinphos, Monocrotophos, Oxydemeton Methyl, Phenthoate, Phorate, Phosphamidon,
Quinalphos, TEPP, and Thiometon.
The following compounds are
moderately toxic (LD50:501 to 5000 mg/kg),
or slightly toxic (LD50: more than 5000
mg/kg)—
Abate, Acephate, Coumaphos,
Crufomate, Famphur, Glyphosate, Malathion, Phenthoate, Primiphos Methyl,
Ronnel, Temephos, Triazophos, and Trichlorphon.
Even in cases where treatment was
begun early with atropine and oximes, mortality in organophosphate poisoning is
gener-ally to the extent of 7 to 12%.
Mode of Action
·
Organophosphates are powerful
inhibitors of acetylcho-linesterase which is responsible for hydrolysing
acetyl-choline to choline and acetic acid after its release and completion of
function (i.e. propagation of action poten-tial). As a result, there is
accumulation of acetylcholine with continued stimulation of local receptors and
eventual paralysis of nerve or muscle.
·
Although organophosphates differ
structurally from acetylcholine, they can bind to the acetylcholinesterase
molecule at the active site and phosphorylate the serine moiety. When this
occurs, the resultant conjugate is infinitely more stable than the
acetylcholine -acetylcho-linesterase conjugate, although endogenous hydrolysis
does occur. Depending on the amount of stability and charge distribution, the
time to hydrolysis is increased. Phosphorylated enzymes degrade very slowly
over days to weeks, making the acetylcholinesterase essentially inactive.
· Once the acetylcholinesterase is phosphorylated, over the next 24 to 48 hours an alkyl group is eventually lost from the conjugate, further exacerbating the situation. As this occurs, the enzyme can no longer spontaneously hydrolyse and becomes permanently inactivated.
·
Apart from acetylcholinesterase,
organophosphates exert powerful inhibitory action over other carboxylic ester
hydrolases such as chymotrypsin, butyrlcholinesterase (pseudocholinesterase),
plasma and hepatic carboxyles- terases, paraoxonases, and other non-specific
proteases.
·
It has been proposed that delayed
peripheral neuropathy caused by organophosphates is due to phosphorylation of
some esterase(s) other than acetylcholinesterase, such as neurotoxic esterase,
also known as neuropathy target esterase (NTE). Neuropathy caused by inhibition
of NTE may develop 2 to 5 weeks after an acute poisoning.
Toxicokinetics
·
Organophosphates can be absorbed by
any route including transdermal, transconjunctival, inhalational, across the GI
and GU mucosa, and through direct injection.
·
hours, but may be delayed upto 12
hours or more in the case of certain compounds (e.g. fenthion, parathion).
Clinical (Toxic) Features
Acute Poisoning:
·
Cholinergic Excess—
– Musscarinic Effects (hollow organ
parasympathetic manifestations): Common manifestations include bronchoconstriction
with wheezing and dyspnoea, cough, pulmonary oedema, vomiting,
diarrhoea,abdominal cramps, increased salivation, lacrima-tion, and sweating,
bradycardia, hypotension, miosis, and urinary incontinence. Some of these can
be remembered by the acronym SLUDGE
—Salivation, Lacrimation,
U rination, Diarrhoea, Gastrointestinal
distress and Emesis.
Excessivesalivation, nausea, vomiting, abdominal cramps, and diarrhoea are
common muscarinic effects, and have been reported even following the cutaneous
absorption of organophosphate. Bradycardia and hypotension occur following
moderate to severe poisoning.
Nicotinic Effects (autonomic ganglionic and somatic
motor effects): Fasciculations, weakness, hypertension, tachycardia, and
paralysis. Muscle weakness, fatiguability, and fasciculations are very common.
Hypertension can occur in up to 20 per cent of patients. Tachycardia is also
common. Cardiac arrhythmias and conduction defects have been reported in
severely poisoned patients. ECG abnormalities may include sinus bradycardia or
tachycardia, atrioventricular and/or intraven-tricular conduction delays,
idioventricular rhythm, multiform premature ventricular extrasystoles,
ventricular tachycardia or fibrillations, torsades de pointes, prolongation of
the PR, QRS, and/or QT intervals, ST-T wave changes, and atrial fibrillation.
·
CNS Effects—Restlessness, headache, tremor, drowsi-ness,
delirium, slurred speech, ataxia, and convulsions. Coma supervenes in the later
stages. In a review of 16 cases of paediatric organophosphate poisoning, all 16
children developed stupor and/or coma. Death usually results from respiratory
failure due to weakness of respiratory muscles, as well as depression of
central respiratory drive. Acute lung injury (non-cardiogenic pulmonary oedema)
is a common manifestation of severe poisoning. Acute respiratory insufficiency,
due to any combination of CNS depression, respiratory paralysis, bronchospasm,
ARDS, or increased bronchial secretions, is the main cause of death in acute
organo-phosphate poisonings.Metabolic acidosis has occurred in severe
poisonings. A characteristic kerosene-like odour is often perceptible in the
vicinity of the patient since the solvent used in many organophosphate
insec-ticides is some petroleum derivative such as aromax.![]()
·
Other points of importance—
––
The Peradeniya Organophosphorus Poisoning (POP) Scale is predictive of
death, necessity for mechanical ventilation, and the required total atro-pine
dose over the first 24 hours. This scale rates 5 clinical variables, each on a
0 to 2 scale: miosis, muscle fasciculations, respirations, bradycardia, and
level of consciousness.
–– In a given case, there may be
either tachy- or brady-cardia; hypo- or hypertension.
––
Miosis while being a characteristic feature, may not be apparent in the
early stages. In fact mydriasis is very often present, and hence treatment
should not be delayed if there is absence of pupillary constric-tion. Blurred
vision may persist for several months.
–– Ocular exposure can result in
systemic toxicity. It can cause persistent miosis in spite of appropriate
systemic therapy, and may necessitate topical atro-pine (or scopolamine)
instillation.
–– Exposure to organophosphate
vapours rapidly produces symptoms of mucous membrane and upper airway
irritation and bronchospasm, followed by systemic symptoms if patients are
exposed to significant concentrations.
–– While respiratory failure is the
commonest cause of death, other causes may contribute including hypoxia due to
seizures, hyperthermia, renal failure, and hepatic failure.
–– Patients with OP poisoning and
QTc prolongation are more likely to develop respiratory failure and have a
worse prognosis than patients with normal QTc intervals. Patients with OP
poisoning who develop PVCs (premature ventricular contractions) are more likely
to develop respiratory failure and have a higher mortality rate than patients
without PVCs.
–– Aspiration of preparations
containing hydrocarbon solvents may cause potentially fatal lipoid
pneu-monitis.
––
An Intermediate Syndrome
sometimes occurs one to four days after poisoning due to long-lasting
cholin-esterase inhibition and muscle necrosis. It is more ![]() common with chlorpyrifos,
dimethoate, monocro-tophos, parathion, sumithion, fenthion, fenitrothion, ethyl
parathion, methyl parathion, diazinon, mala-thion, and trichlorfon. Main
features include muscle weakness and paralysis characterised by motor cranial
nerve palsies, weakness of neck flexor and proximal limb muscles, and acute
respiratory paresis. Paralytic signs include inability to lift the neck or sit
up, ophthalmoparesis, slow eye movements, facial weakness, difficulty
swallowing, limb weakness (primarily proximal), areflexia, respiratory
paralysis, and death. It may be due to inadequate treatment of the acute
episode especially involving subtherapeutic administration of oximes or
inadequate assisted ventilation. Several investigators have proposed that intermediate
syndrome may develop as a result of several factors: inadequate oxime therapy,
the dose and route of exposure, the chemical structure of the organophosphates,
the time to initiation of therapy, and possibly efforts to decrease absorption
or enhance elimination of the organophosphates. Once it sets in, the
intermediate syndrome will have to be managed by supportive measures, since it
does not respond to oximes or atropine.
common with chlorpyrifos,
dimethoate, monocro-tophos, parathion, sumithion, fenthion, fenitrothion, ethyl
parathion, methyl parathion, diazinon, mala-thion, and trichlorfon. Main
features include muscle weakness and paralysis characterised by motor cranial
nerve palsies, weakness of neck flexor and proximal limb muscles, and acute
respiratory paresis. Paralytic signs include inability to lift the neck or sit
up, ophthalmoparesis, slow eye movements, facial weakness, difficulty
swallowing, limb weakness (primarily proximal), areflexia, respiratory
paralysis, and death. It may be due to inadequate treatment of the acute
episode especially involving subtherapeutic administration of oximes or
inadequate assisted ventilation. Several investigators have proposed that intermediate
syndrome may develop as a result of several factors: inadequate oxime therapy,
the dose and route of exposure, the chemical structure of the organophosphates,
the time to initiation of therapy, and possibly efforts to decrease absorption
or enhance elimination of the organophosphates. Once it sets in, the
intermediate syndrome will have to be managed by supportive measures, since it
does not respond to oximes or atropine.
–– A Delayed Syndrome sometimes occurs 1 to 4 weeks after poisoning due
to nerve demyelination, and is characterised by flaccid weakness and atrophy of
distal limb muscles, or spasticity and ataxia. A mixed sensory-motor neuropathy
usually begins in the legs, causing burning or tingling, then weakness. This
syndrome also does not respond to either oximes or atropine. Severe cases
progress to complete paralysis, impaired respiration and death. The nerve
damage of organophosphate-induced delayed neuropathy is frequently permanent.
The mechanism appears to involve phosphorylation of esterases in peripheral
nervous tissue and results in a “dying back” pattern of axonal degeneration.
Organophosphates that have been associated with delayed neuropathy in humans
include chlorophos, chlorpyrifos, dichlorvos, dipterex, ethyl para-thion,
fenthion, isofenphos, leptophos, malathion, mecarbam, merphos, methamidophos,
mipafox, trichlorofon, trichloronate, and TOCP (tri-ortho-cresyl phosphate).
––
Parathion ingestion is sometimes associated with haemorrhagic
pancreatitis which can termi-nate fatally. Diazinon has also been implicated.
Haemoperfusion is said to be
beneficial if this occurs.
–– Patients poisoned with highly
lipid soluble OPs such as fenthion have rarely developed extrapy-ramidal
effects including dystonia, resting tremor, cog-wheel rigidity, and
choreoathetosis. These effects began 4 to 40 days after acute OP poisoning and
spontaneously resolved over 1 to 4 weeks in survivors.
––
It is important to note that children may have different predominant
signs of organophosphate poisoning than adults. In one study of children
poisoned by organophosphate or carbamate compounds, the major signs and
symptoms were CNS depression, stupor, flaccidity, dyspnoea, and coma. Other
classical signs of organophosphate poisoning such as miosis, fasciculations,
brady-cardia, excessive salivation and lacrimation, and gastrointestinal
symptoms were infrequent.
–– Bradypnoea sometimes occurs.
Respiratory rates of less than 8/minute are not unusual. Snoring prior to fatal
overdose has been reported and is likely due to a failure to maintain the
patency of the upper airway. Gurgling may occur due to accumulation of
pulmonary oedema fluid. Non-cardiogenic pulmonary oedema is an infrequent, but
severe, complication of overdose and is generally abrupt in onset (immediate-2
hours). Manifestations include rales, pink frothy sputum, significant hypoxia,
and bilateral fluffy infiltrates on chest X-ray. Some patients require
mechanical ventilation. Resolution of symptoms usually occurs rapidly with
supportive care alone, within hours to 1 to 2 days.
Chronic Poisoning:
·
It usually occurs as an occupational hazard in
agriculturists, especially those who are engaged in pesticide spraying of
crops. Route of exposure is usually inhalation or contamination of skin. The
following are the main features—
·
Polyneuropathy: paraesthesias, muscle cramps,
weak-ness, gait disorders.
·
CNS Effects : drowsiness, confusion,
irritability, anxiety.
·
Sheep Farmer’s Disease : psychiatric
manifestationsencountered in sheep farmers involved in long-term sheep-dip
operations.
·
Organophosphate poisoning has been associated with a variety
of subacute or delayed onset chronic neuro-logical, neurobehavioural, or
psychiatric syndromes. One author has termed these “chronic
organophos-phate-induced neuropsychiatric disorder; (COPND) and noted that the
standard hen neurotoxic esterase test is not sufficient to detect which OPs can
cause this condition.
Diagnosis
1. Depression of cholinesterase
activity:
If the RBC cholinesterase level is
less than 50% of normal, it indicates organophosphate toxicity. RBC
cholinesterase levels are more reliable in diagnosing organophosphate poisoning
than serum cholinesterase. –– Disadvantages—
-- Normal cholinesterase level is
based on popula-tion estimates and there is a wide distribution in the
definition of normal. A person with a “high normal” level may become
symptomatic with a “low normal” activity.
--Several individuals do not seem to
possess a known baseline level.
--A very low cholinesterase level
does not always correlate with clinical illness.
--False depression of RBC
cholinesterase level is seen in pernicious anaemia, haemoglobinopathies,
anti-malarial treatment, and blood collected in oxalate tubes. Elevated levels
may be seen with reticulocytosis due to anaemias, haemorrhage, or treatment of
megaloblastic or pernicious anaemias.
Depression of plasma cholinesterase
level (to less than 50%) is a less reliable indicator of organophosphate
toxicity, but is easier to assay and more commonly done. Depressions in excess
of 90% may occur in severe poisonings, and is usually associated with
mortality.
–– Because it is a liver protein,
plasma cholinesterase activity is depressed in cirrhosis, neoplasia,
malnu-trition, and infections, some anaemias, myocardial infarction, and
chronic debilitating conditions.
–– Certain drugs such as sucinyl
choline, lignocaine, codeine, and morphine, thiamine, ether, and chlo-roquine
can also depress its activity.
–– Studies have demonstrated that
RBC cholinesterase levels may be significantly higher in pregnant women than in
nonpregnant controls, while plasma cholinesterase levels are generally lower
during pregnancy. These levels revert to normal by six weeks postpartum.
–– The organophosphates phosdrin and
chlorpyrifos may selectively inhibit plasma pseudocholines-terase, while
phosmet and dimethoate may selec-tively inhibit red blood cell cholinesterase.
For the purpose of estimation of
cholinesterase level, blood should be collected only in heparinised tubes.
Alternatively, samples can be frozen. Plasma cholin-esterase usually recovers
in a few days or weeks; red blood cell cholinesterase recovers in several days
to 4 months depending on severity of depression.
2. P-Nitrophenol Test: P-nitrophenol is a metabolite of
someorganophosphates (e.g. parathion, ethion), and is excreted in the urine.
Steam distill 10 ml of urine and collect the distillate. Add sodium hydroxide
(2 pellets) and heat on
water bath for 10 minutes.
Production of yellow colour indicates the presence of p-nitrophenol. The test
can also be done on vomitus or stomach contents.
3. Thin Layer Chromatography (TLC):
The presence of anorganophosphate in a lavage, or vomit, or gastric aspirate
sample can also be determined by TLC. The sample is extracted twice with 5 ml
of petroleum ether, and the extract is washed with distilled water. It is then
dried in steam compressed air, reconstituted in methanol, and spotted on silica
gel-coated TLC plate along with the standard and run in a mixture of petroleum
ether and methanol (25 : 1). After the solvent has travelled a considerable
distance, the plate is dried and exposed to iodine vapour. The RF is compared
with that of the standard.
4. Ancillary Investigations:
·
There may be evidence of leukocytosis (with relatively
normal differential count), high haematocrit, anion gap acidosis,
hyperglycaemia.
·
In every case, monitor electrolytes, ECG and serum
pancreatic isoamylase levels in patients with significant poisoning. Patients
who have increased serum amylase levels and those who develop a prolonged QTc
interval or PVCs are more likely to develop respiratory insuf-ficiency and have
a worse prognosis. If pancreatitis is suspected, an abdominal CT-scan can be
performed to evaluate diffuse pancreatic swelling.
·
If respiratory tract irritation is present, monitor chest
X-ray. Many organophosphate compounds are found in solution with a variety of
hydrocarbon-based solvents. Aspiration pneumonitis may occur if these products
are aspirated into the lungs. Bronchopneumonia may develop as a complication of
organophosphate-induced pulmonary oedema.
·
High performance thin layer chromatography (HPLC) technique
can be used to identify several organophos-phate compounds in human serum.![]()
Treatment
Determine
plasma or red blood cell cholinesterase activities. Depression in excess of 50
per cent of baseline is generally associated with severe symptoms (vide supra).
Acute Poisoning:
a. Decontamination:
––
If skin spillage has occurred, it is imperative that the patient be stripped
and washed thoroughly with soap and water.
--
Shower is preferable. Make the patient stand (if he is able to) under the
shower, or seated in a chair.
--
Wash with cold water for 5 minutes from head to toe using non-germicidal soap.
Rinse hair well.
--
Repeat the wash and rinse procedure with warm water.
--
Repeat the wash and rinse procedure with hot water.
--
Treating personnel should protect themselves with water-impermeable gowns,
masks with eye shields, and shoe covers. Latex and vinyl gloves provide
inadequate protection, unless a double pair is used.
––
If ocular exposure has occurred, copious eye irriga-tion should be done with
normal saline or Ringer’s solution. If these are not immediately available, tap
water can be used.
––
In the case of ingestion, stomach wash can be done, though this is often
unnecessary because the patient would have usually vomited several times by the
time he is brought to hospital. Activated charcoal can be administered in the
usual way.
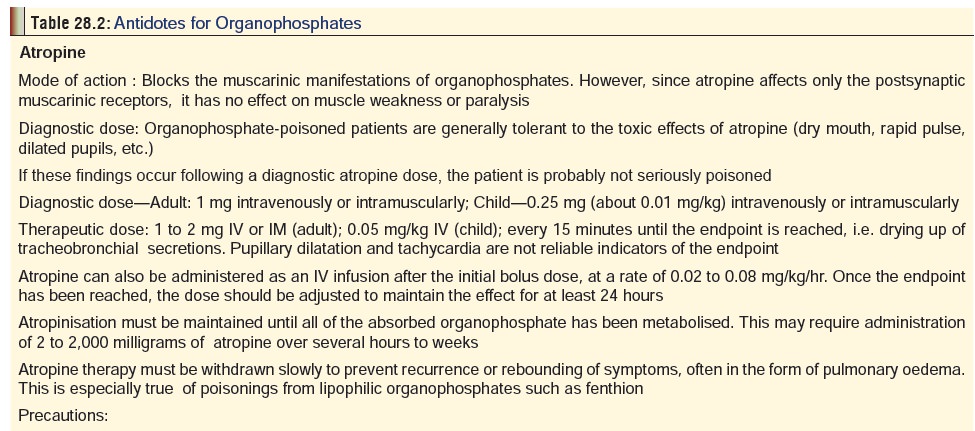
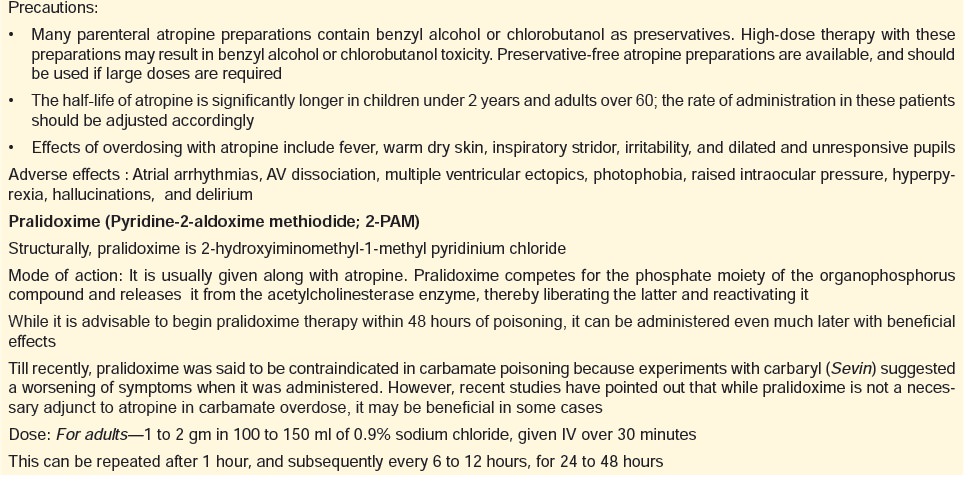
b. Antidotes:
–– Atropine—It is a competitive antagonist of
acetyl- choline at the muscarinic postsynaptic membrane and in the CNS, and
blocks the muscarinic mani- festations of organophosphate poisoning.
–– Oximes—The commonest is pralidoxime
(pyridine- 2-aldoxime methiodide), which is a nucleophilic oxime that helps to
regenerate acetylcholinesterase at muscarinic, nicotinic, and CNS sites.
Actually, human studies have not conclusively substantiated the benefit of
oxime therapy in acute organophos- phate poisoning, but they are widely used.
Most authors advocate the continued use of pralidoxime in the clinical setting
of severe organophosphate poisoning.
The antidotes for organophosphates have been discussed together in detail in Table 28.2.
c. Supportive Measures:
–– Administer IV fluids to replace losses.
–– Maintain airway patency and oxygenation.
Suction secretions. Endotracheal intubation and mechanical ventilation may be
necessary. Monitor pulse oxim-etry or arterial blood gases to determine need
for supplemental oxygen.
–– Oxygenation/intubation/positive pressure
ventila-tion: To minimise barotrauma and other compli- cations, use the lowest
amount of PEEP possible while maintaining adequate oxygenation. Use of smaller
tidal volumes (6 ml/kg) and lower plateau pressures (30 cm water or less) has
been associated with decreased mortality and more rapid weaning from mechanical
ventilation in patients with ARDS.
–– The following drugs are contraindicated: parasym- pathomimetics, phenothiazines, antihistamines, and opiates. Do not administer succinylcholine (suxamethonium) or other cholinergic medica-tions. Prolonged neuromuscular blockade may result when succinylcholine is administered after organophosphate exposure.
––
Treat convulsions with benzodiazepines or barbi-turates.
––
Antibiotics are indicated only when there is evidence of infection.
––
Haemoperfusion, haemodialysis, and exchange transfusion have not been shown to
affect outcome or duration of toxicity in controlled trials of organo-phosphate
poisoning.
d. Prevention of Further Exposure: After the patient hasrecovered, he
should not be re-exposed to organophos-phates for at least a few weeks since he
is likely to suffer serious harm from a dose that normally would be harmless,
owing to alteration of body chemistry. Following acute poisoning, patients
should be precluded from further organophosphate exposure until sequential RBC
cholinesterase (AChE) levels have been obtained and confirm that AChE activity
has reached a plateau.
Plateau has been obtained when
sequential determina-tions differ by no more than 10%. This may take 3 to 4
months following severe poisoning.
e. Treatment of Pregnant Victim: Therapeutic choicesduring pregnancy
depend upon specific circumstances such as stage of gestation, severity of
poisoning, and clinical signs of mother and foetus. The mother must be treated
adequately to treat the foetus. A severely poisoned patient with a late
gestation viable foetus may be a candidate for emergency Caesarean section. The
foetus may require intensive care after birth.
–– Pralidoxime chloride is
recommended for use in the pregnant patient to counteract muscle weakness.
–– Glycopyrrolate: Unlike atropine,
glycopyrrolate usually does not readily cross the placenta and would not
directly affect foetal poisoning. However, the foetus may be best served by
treating the mother to retain good respiratory function and foetal
oxygen-ation.
Chronic Poisoning:
·
Removal of the patient from the
source of exposure.
·
Supportive and symptomatic measures.
Autopsy Features
External—
·
Characteristic odour (garlicky or
kerosene-like).
·
Frothing at mouth and nose.
· Cyanosis of extremities.
·
Constricted pupils.
Internal—
·
Congestion of GI tract; garlicky or
kerosene-like odour of contents.
·
Pulmonary and cerebral oedema.
·
Generalised visceral congestion.
Related Topics