organic reactions - Nucleophiles and elctrophiles | 11th Chemistry : UNIT 12 : Basic concepts of organic reactions
Chapter: 11th Chemistry : UNIT 12 : Basic concepts of organic reactions
Nucleophiles and elctrophiles
Nucleophiles
and elctrophiles
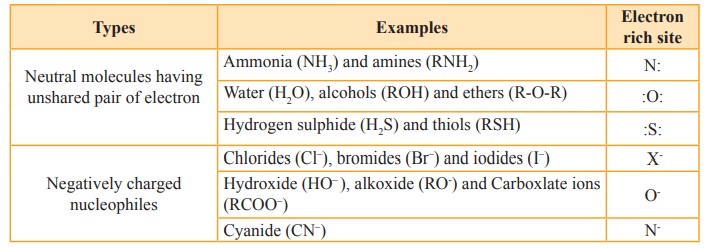
Nucleophiles
are reagents that has high affinity for electro positive centers. They possess
an atom has an unshared pair of electrons, and hence it is in search for an
electro positive centre where it can have an opportunity to share its elections
to form a covalent bond, and gets stabilised. They are usually negatively
charged ions or electron rich neutral molecules (contains one or more lone pair
of electrons). All Lewis bases act as nucleophiles.
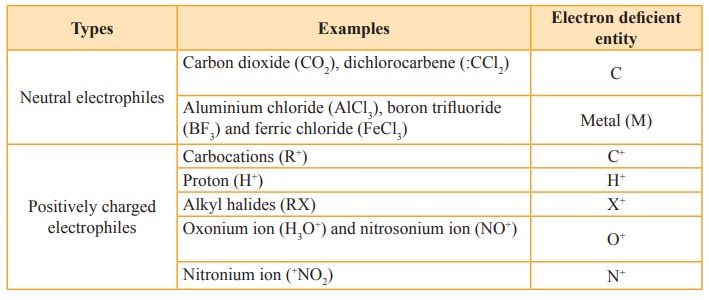
Electrophiles
are reagents that are attracted towards negative charge or electron rich
center. They are either positively charged ions or electron deficient neutral
molecules. All Lewis acids act as electrophiles. Neutral molecules like SnCl4
can also act as an electrophile, as it has vacant d-orbitals which can
accommodate the electrons from others.
Related Topics