Chapter: 11th Chemistry : UNIT 12 : Basic concepts of organic reactions
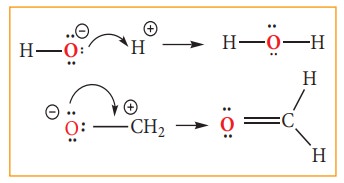
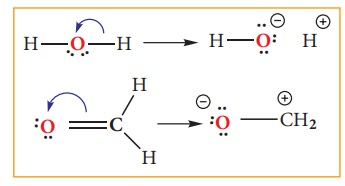
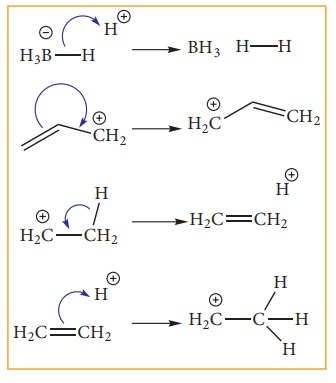
Electron movement in organic reactions
Electron
movement in organic reactions
All
organic reactions can be understood by following the electron movements, i.e.
the electron redistribution during the reaction. The electron movement depends
on the nature of the substrate, reagent and the prevailing conditions. The flow
of electrons is represented by curved arrows which show how electrons move as
shown in the figure. These electron movements result in breaking or formation
of a bond (sigma or pi bond). The movement of single electron is indicated by a
half -headed curved arrows.
There
are three types of electron movement viz.,
·
lone
pair becomes a bonding pair.
·
bonding
pair becomes a lone pair
·
a
bond breaks and becomes another bond.
Type 1: A lone pair to a bonding pair
Type 2: A bonding pair to a lone pair
Type 3: A bonding pair to an another bonding pair
Related Topics