Chapter: 11th Chemistry : UNIT 12 : Basic concepts of organic reactions
Electron displacement effects in co-valent bonds
Electron
displacement effects in co-valent bonds
Some
of the properties of organic molecules such as stability, reactivity, basicity
etc., are affected by the displacement of electrons that takes place in its
covalent bonds. This movement can be influenced by either the atoms/groups
present in close proximity to the bond or when a reagent approaches a molecule.
The displacement effects can either be permanent or a temporary. In certain
cases, the electron displacement due to an atom or a substituent group present
in the molecule cause a permanent polarisation of the bond and it leads to
fission of the bond under suitable conditions. The electron displacements are
catagorised into inductive effect (I), resonance effect (R), electromeric
effect (E) and hyper conjugation.
Inductive effect (I)
Inductive
effect is defined as the change in the polarisation of a covalent bond due to
the presence of adjacent bonds, atoms or groups in the molecule. This is a
permanent phenomenon.
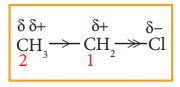
Let
us explain the inductive effect by considering ethane and ethylchloride as
examples. The C-C bond in ethane is non polar while the C-C bond in ethyl
chloride is polar. We know that chlorine is more electronegative than carbon,
and hence it attracts the shared pair of electron between C-Cl in ethyl
chloride towards itself. This develops a slight negative charge on chlorine and
a slight positive charge on carbon to which chlorine is attached. To compensate
it, the C1 draws the shared pair of electron between itself and C2.
This polarisation effect is called inductive effect. This effect is greatest
for the adjacent bonds, but they also be felt farther away. However, the
magnitude of the charge separation decreases rapidly, as we move away from C1
and is observed maximum for 2 carbons and almost insignificant after 4 bonds
from the active group.
It
is important to note that the inductive effect does not transfer electrons from
one atom to another but the displacement effect is permanent. The inductive
effect represents the ability of a particular atom or a group to either withdraw
or donate electron density to the attached carbon. Based on this ability the
substituents are classified as +I groups and -I groups. Their ability to
release or withdraw the electron through sigma covalent bond is called +I
effect and -I effect respectively.
Highly
electronegative atoms and atoms of groups which are carry a positive charge are
electron withdrawing or -I group
Example: -F, -Cl, -COOH, -NO2,
NH2
Higher
the electronegativity of the substitutent, greater is the -I effect. The order
of the –I effect of some groups are given below.
NH3+>
NO2> CN > SO3H > CHO > CO > COOH > COCl
> CONH2> F > Cl > Br > I > OH > OR > NH2>
C6H5> H
Highly
electropositive atoms and atoms are groups which carry a negative charge are
electron donating or +I groups.
Example.
Alkali metals, alkyl groups such as methyl, ethyl, negatively charged groups
such as CH3O–, C2H5O–,
COO– etc
Lesser
the electronegativity of the elements, greater is the +I effect. The relative
order of +I effect of some alkyl groups is given below
–C(CH3)3>
–CH(CH3)2>–CH2CH3>–CH3
Let
us understand the influence of inductive effect on some properties of organic
compounds.
Reactivity:
When
a highly electronegative atom such as halogen is attached to a carbon then it
makes the C-X bond polar. In such cases the -I effect of halogen facilitates
the attack of an incoming nucleophile at the polarised carbon, and hence
increases the reactivity.
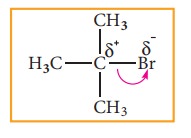
If
a -I group is attached nearer to a carbonyl carbon, it decreases the
availability of electron density on the carbonyl carbon, and hence increases
the rate of the nucelophilic addition reaction.
Acidity of carboxylic acids:
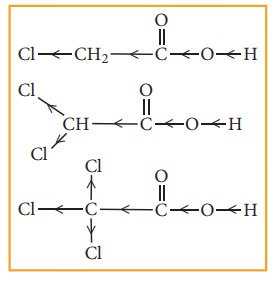
When a halogen atom is attached to the carbon which is nearer to the carboxylic acid group, its -I effect withdraws the bonded electrons towards itself and makes the ionisation of H+ easy. The acidity of various chloro acetic acid is in the following order. The strength of the acid increases with increase in the -I effect of the group attached to the carboxyl group.
Trichloro
acetic acid > Dichloro acetic acid > Chloro acetic acid > acetic acid
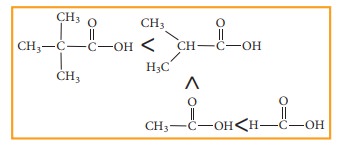
Similarly,
the following order of acidity in the carboxylic acids is due to the +I effect
of alkyl group.
Electrometric effect (E)
Electromeric
is a temporary effect which operates in unsaturated compounds (containing
>C=C<, >C=O, etc...) in the presence of an attacking reagent.
Let
us consider two different compounds (i) compounds containing carbonyl group
(>C=O) and (ii) unsaturated compounds such as alkenes (>C=C< ).
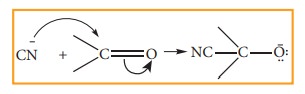
When
a nucleophile approaches the carbonyl compound, the π electrons between C and O is instantaneously shifted to the more
electronegative oxygen. This makes the carbon electron deficient and thus
facilitating the formation of a new bond between the incoming nucleophile and
the carbonyl carbon atom.
On
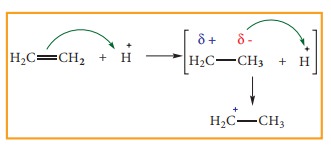
the other hand when an electrophile such as H+ approaches an alkene molecule,
the π electrons are instantaneously
shifted to the electrophile and a new bond is formed between carbon and
hydrogen. This makes the other carbon electron deficient and hence it acquires
a positive charge.
The
electromeric effect, is denoted as E effect. Like the inductive effect, the
electromeric effect is also classified as +E and -E based on the direction in
which the pair of electron is transfered to form a new bond with the attacking
agent.
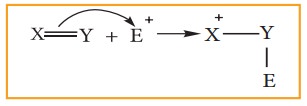
When
the π electron is transferred
towards the attacking reagent, it is called + E (positive electromeric) effect.
The
addition of H+ to alkene as shown above is an example of +E effect.
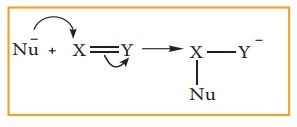
When
the π electron is transfered away
from the attacking reagent, it is called, -E (negative electromeric) effect
The
attack of CN- on a carbonyl carbon, as shown above, is an example of -E effect.
Resonance or Mesomeric effect
The
resonance is a chemical phenomenon which is observed in certain organic
compounds possessing double bonds at a suitable position. Certain organic
compounds can be represented by more than one structure and they differ only in
the position of bonding and lone pair of electrons. Such structures are called
resonance structures (canonical structures) and this phenomenon is called
resonance. This phenomenon is also called mesomerism or mesomeric effect.
For
example, the structure of aromatic compounds such as benzene and conjugated
systems like 1,3-butadiene cannot be represented by a single structure, and
their observed properties can be explained on the basis of a resonance hybrid.
In
1,3 buta diene, it is expected that the bond between C1-C2
and C3 –C4 should be shorter than that of C2-C3,
but the observed bond lengths are of same. This property cannot be explained by
a simple structure in which two π bonds localised between C1-C2
and C3 –C4. Actually the π electrons are delocalised as
shown below.
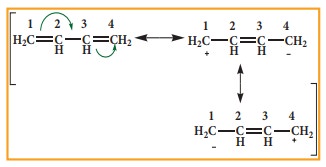
These
resonating structures are called canonical forms and the actual structure lies
between these three resonating structures, and is called a resonance hybrid.
The resonance hybrid is represented as below.
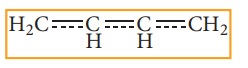
Similar
to the other electron displacement effect, mesomeric effect is also classified
into positive mesomeric effect (+M or +R) and negative mesomeric effect (-M of
-R) based on the nature of the functional group present adjacent to the
multiple bond.
Positive Mesomeric Effect:
Positive
resonance effect occurs, when the electrons move away from substituent attached
to the conjugated system. It occurs, if the electron releasing substituents are
attached to the conjugated system. In such cases, the attached group has a
tendency to release electrons through resonance. These electron releasing
groups are usually denoted as +R or +M groups.
Examples : -OH, -SH, -OR,-SR, -NH2,
-O-etc...
Negative Mesomeric Effect
Negative resonance effect occurs, when the electrons move towards the substituent attached to the conjugated system. It occurs if the electron withdrawing substituents are attached to the conjugated system.
In such cases, the attached group has a
tendency to withdraw electrons through resonance. These electron withdrawing
groups are usually denoted as -R or -M groups. Examples : NO2, >C=O, -COOH,-C≡N etc
Resonance
is useful in explaining certain properties such as acidity of phenol. The
phenoxide ion is more stabilised than phenol by resonance effect(+M effect) and
hence resonance favours ionisation of phenol to form H+ and shows
acidity.
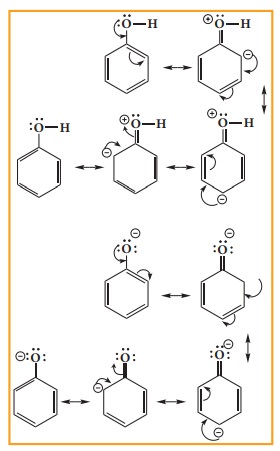
The
above structures shows that there is a charge separation in the resonance
structure of phenol which needs energy, where as there is no such hybrid
structures in the case of phenoxide ion. This increased stability accounts for
the acidic character of phenol.
Hyper conjugation
The
delocalisation of electrons of σ bond is called as hyper conjugation. It is a
special stabilising effect that results due to the interaction of electrons of
a σ-bond (usually C-H or C-C) with the adjacent, empty non-bonding p-orbital or an
anti-bonding σ* or π*-orbitals resulting in an extended
molecular orbital. Unlike electromeric effect, hyper conjugation is a permanent
effect.
It
requires an α-CH group or a lone pair on atom like N, O adjacent to a π bond
(sp2 hybrid carbon). It occurs by the overlapping of the σ-bonding
orbital or the orbital containing a lone pair with the adjacent π-orbital or
p-orbital.
Example 1:
In
propene, the σ-electrons of C-H bond
of methyl group can be delocalised into the π-orbital
of doubly bonded carbon as represented below.
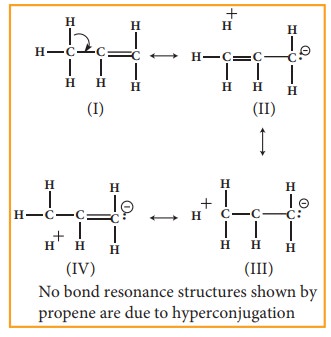
In
the above structure the sigma bond is involved in resonance and breaks in order
to supply electrons for delocalisation giving rise to 3 new canonical forms. In
the contributing canonical structures: (II), (III) & ![]()
![]() (IV) of propene, there is no bond between an α-carbon and
one of the hydrogen atoms. Hence the hyperconjugation is also known as “no bond
resonance” or “Baker-Nathan effect”. The structures (II), (III) & (IV) are
polar in nature.
(IV) of propene, there is no bond between an α-carbon and
one of the hydrogen atoms. Hence the hyperconjugation is also known as “no bond
resonance” or “Baker-Nathan effect”. The structures (II), (III) & (IV) are
polar in nature.
Example 2:
Hyper
conjugation effect is also observed when atoms / groups having lone pair of
electrons are attached by a single bond, and in conjugation with a π bond. The lone pair of electrons
enters into resonance and displaces π
electrons resulting in more than one structure
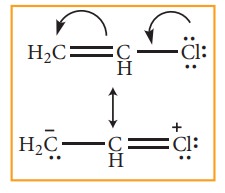
Example 3:
When
electronegative atoms or group of atoms are in conjugation with a π -bond,they
pull π - electrons from the multiple bond.
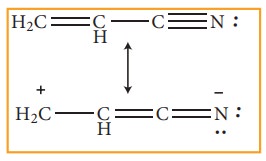
In
case of carbocations, greater the number of alkyl groups attached to the carbon
bearing positive charge, greater is number of the hyper conjugate structure.
thus the stability of various carbocations decreases in the order
3º
Carbocation > 2 º Carbocation > 1 º Carbocation
Related Topics