Chapter: Clinical Anesthesiology: Clinical Pharmacology: Neuromuscular Blocking Agents
Neuromuscular Transmission
Neuromuscular Blocking Agents
Skeletal muscle relaxation can be
produced by deep inhalational anesthesia, regional nerve block, or
neuromuscular blocking agents (commonly called muscle relaxants). In 1942, Harold Griffith pub-lished the results
of a study using an extract of cu-rare (a South American arrow poison) during
anes-thesia. Following the introduction of succinylcholine as a “new approach
to muscular relaxation,” these agents rapidly became a routine part of the
anesthe-siologist’s drug arsenal. However, as noted by Beecher and Todd in
1954: “[m]uscle relaxants giv-en inappropriately may provide the surgeon with
optimal [operating] conditions in . . . a patient [who] is paralyzed but not
anesthetized—a state [that] is wholly unacceptable for the patient.” In other
words, muscle relaxation does not ensure un-
consciousness, amnesia, or analgesia.
Neuromuscular Transmission
Association between a motor neuron and a
mus-cle cell occurs at the neuromuscular junction (Figure 11–1). The cell membranes
of the neuron and muscle fiber are separated by a narrow (20-nm) gap, the
synaptic cleft. As a nerve’s action potential depo-larizes its terminal, an
influx of calcium ions through voltage-gated calcium channels into the nerve
cyto-plasm allows storage vesicles to fuse with the ter-minal plasma membrane
and release their contents
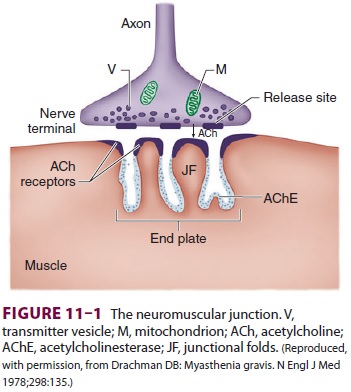
(acetylcholine [ACh]). The ACh molecules
diffuse across the synaptic cleft to bind with nicotinic cholin-ergic receptors
on a specialized portion of the muscle membrane, the motor end-plate. Each
neuromuscu-lar junction contains approximately 5 million of these receptors,
but activation of only about 500,000 recep-tors is required for normal muscle
contraction.
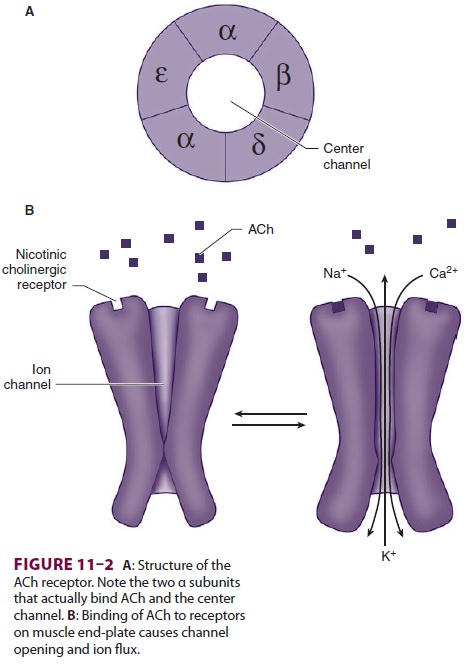
The structure of ACh receptors varies in
differ-ent tissues and at different times in development. Each ACh receptor in
the neuromuscular junction normally consists of five protein subunits; two α subunits; and
single β, δ, and ε subunits. Only the two identical α subunits are
capable of binding ACh molecules. If both binding sites are occupied by ACh, a
conformational change in the subunits briefly (1 ms) opens an ion channel in
the core of the recep-tor (Figure 11–2). The channel will not open if ACh
binds on only one site. In contrast to the normal (ormature) junctional ACh
receptor, another isoform contains a γ subunit instead of the ε subunit. This
isoform is referred to as the fetal or immature recep-tor because it is in the form
initially expressed in fetal muscle. It is also often referred to as
extrajunc-tional because, unlike the mature isoform, it may be located anywhere
in the muscle membrane, inside or outside the neuromuscular junction when
expressed in adults.
Cations flow through the open ACh
receptor channel (sodium and calcium in; potassium out), generating an end-plate potential. The contents of a
single vesicle, a quantum of ACh (104
molecules per quantum), produce a miniature end-plate potential. The number of
quanta released by each nerve impulse, normally at least 200, is very
sensi-tive to extracellular ionized calcium concentration; increasing calcium
concentration increases the
number of quanta released. When enough
recep-tors are occupied by ACh, the end-plate potential will be sufficiently
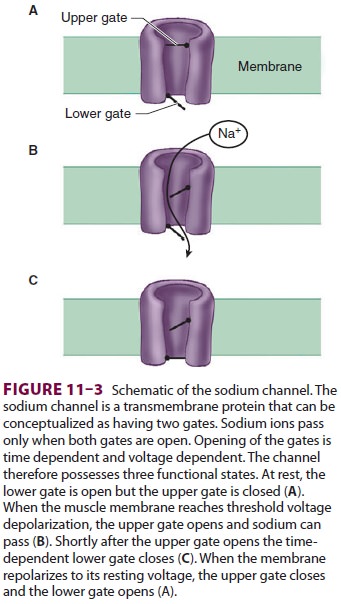
strong to depolarize the perijunc-tional membrane. Voltage-gated sodium
channels within this portion of the muscle membrane open when a threshold
voltage is developed across them, as opposed to end-plate receptors that open
when ACh is applied ( Figure 11–3). Perijunctional areas of muscle
membrane have a higher density of these sodium channels than other parts of the
membrane. The resulting action potential propagates along the muscle membrane
and T-tubule system, opening sodium channels and releasing calcium from the
sarcoplasmic reticulum. This intracellular calcium allows the contractile
proteins actin and myosin to interact, bringing about muscle contraction. The
amount of ACh released and the number of
recep-tors subsequently activated will normally far exceed the minimum required
for the initiation of an action potential. The nearly 10-fold margin of safety
is lost in Eaton–Lambert myasthenic syndrome (decreased release of ACh) and
myasthenia gravis (decreased number of receptors).
ACh is rapidly hydrolyzed into acetate
and choline by the substrate-specific enzyme acetylcho-linesterase. This enzyme (also called specific
cholin-esterase or true cholinesterase) is embedded into the motor end-plate
membrane immediately adjacent to the ACh receptors. After unbinding ACh, the
recep-tors’ ion channels close, permitting the end-plate to repolarize. Calcium
is resequestered in the sarco-plasmic reticulum, and the muscle cell relaxes.
Related Topics