Chapter: Clinical Anesthesiology: Clinical Pharmacology: Neuromuscular Blocking Agents
DepolarizingMuscle Relaxants: Succinylcholine
DepolarizingMuscle Relaxants
SUCCINYLCHOLINE
The only depolarizing muscle relaxant in
clinical usetoday is succinylcholine.
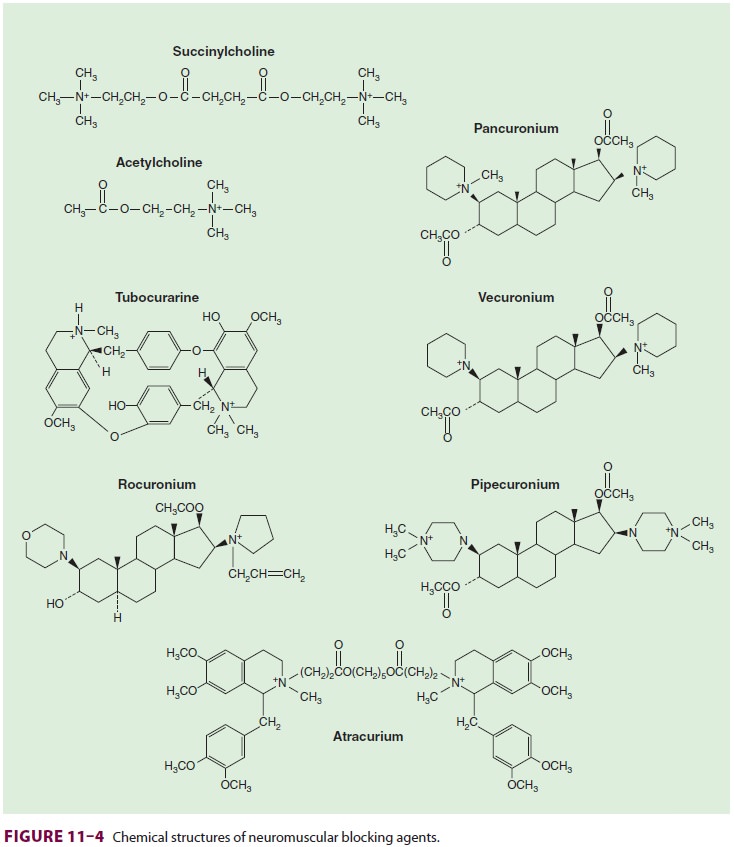
Physical Structure
Succinylcholine—also called
diacetylcholine or suxamethonium—consists of two joinedACh molecules ( Figure 11–4). This structure under-lies
succinylcholine’s mechanism of action, side effects, and metabolism.
Metabolism & Excretion
The popularity of succinylcholine is due to its rapid onset of action (30–60 s) and short duration of action (typically less than 10 min). Its rapid onset of action relative to other neuromuscular blockers is largely due to the relative overdose that is usually administered. Succinylcholine, like all neuromuscular blockers, has a small volume of distribution due to its very low lipid solubility, and this also underlies a rapid onset of action. As succinylcholine enters the circulation, most of it is rapidly metabolized by pseudocholinesterase into succinylmonocholine. This process is so efficient that only a small fraction of the injected dose ever reaches the neuromuscular junction. As drug lev-els fall in blood, succinylcholine molecules diffuse away from the neuromuscular junction, limiting the duration of action. However, this duration of action can be prolonged by high doses, infusion of succinylcholine, or abnormal metabolism. The latter may result from hypothermia, reduced pseu-docholinesterase levels, or a genetically aberrant enzyme. Hypothermia decreases the rate of hydro-lysis. Reduced levels of pseudocholinesterase (mea-sured as units per liter) accompany pregnancy, liver disease, renal failure, and certain drug therapies (Table 11–3). Reduced pseudocholinesterase lev-els generally produce only modest prolongation of succinylcholine’s actions (2–20 min).
One in 25-30 patients of European
extraction is a heterozygote with one normal and one abnormal (atypical)
pseudocholinesterase gene, resulting in a slightly prolonged block (20–30 min).
Even fewer (1 in 3000) patients have two copies of the most prevalent abnormal
gene (homozygous atypical) that produce an enzyme with little or no affinity
for succinylcholine. In contrast to the doubling or tripling of blockade
duration seen in
patients with low enzyme levels or
heterozygous atypical enzyme, patients with homozygous atypi-cal enzyme will
have a very long blockade (eg, 4–8 h)
following administration of succinylcholine. Of the recognized abnormal
pseudocholinesterase genes, the dibucaine-resistant (variant) allele, which
produces an enzyme with 1/100 of normal affinity for succinylcholine, is the
most common. Other variants include fluoride-resistant and silent (no activity)
alleles.
Dibucaine, a local anesthetic, inhibits
normal pseudocholinesterase activity by 80%, but inhib-its atypical enzyme
activity by only 20%. Serum from an individual who is heterozygous for the atypical
enzyme is characterized by an intermediate 40% to 60% inhibition. The
percentage of inhibi-tion of pseudocholinesterase activity is termed the dibucaine number. A patient with normal
pseu-docholinesterase has a dibucaine number of 80; a homozygote for the most
common abnormal allele will have a dibucaine number of 20. The dibucaine number
measures pseudocholinesterase function, not the amount of enzyme. Therefore,
adequacy of pseudocholinesterase can be determined in the laboratory
quantitatively in units per liter (a minor factor) and qualitatively by
dibucaine number (the major factor). Prolonged paralysis from succinyl-choline
caused by abnormal pseudocholinester-ase (atypical cholinesterase) should be
treated with continued mechanical ventilation and seda-tion until muscle
function returns to normal by clinical signs. Such unsedated patients do NOT
appreciate unnecessary, repetitive use of nerve stimulation when all members of
a department come by to confirm the diagnosis.
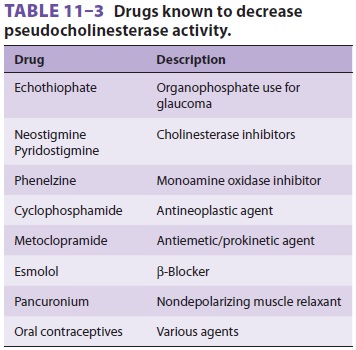
Drug Interactions
The effects of muscle relaxants can be
modified by concurrent drug therapy (Table 11–4). Succinyl-choline is involved in
two interactions deserving special comment.
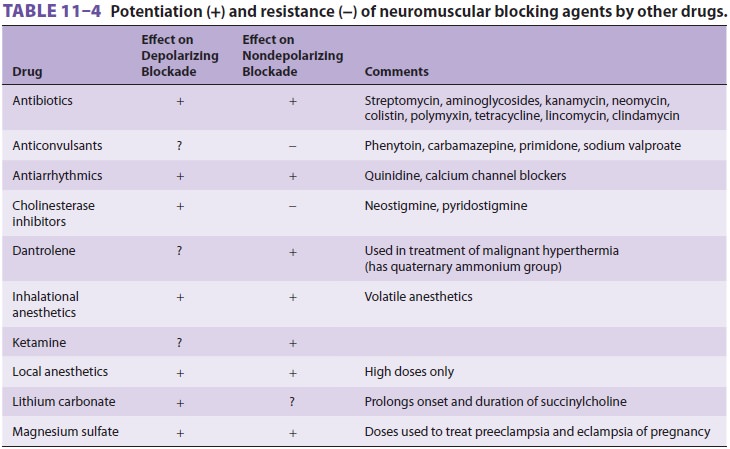
A. Cholinesterase Inhibitors
Although cholinesterase inhibitors reverse non-depolarizing paralysis, they markedly prolong a depolarizing phase I block by two mechanisms. By inhibiting acetylcholinesterase, they lead to a higher ACh concentration at the nerve terminal, which intensifies depolarization. They also reduce the hydrolysis of succinylcholine by inhibiting pseu-docholinesterase. Organophosphate pesticides, for example, cause an irreversible inhibition of acetyl-cholinesterase and can prolong the action of succi-nylcholine by 20–30 min. Echothiophate eye drops, used in the past for glaucoma, can markedly prolong succinylcholine by this mechanism.
B. Nondepolarizing Relaxants
In general, small doses of
nondepolarizing relaxants antagonize a depolarizing phase I block. Because the
drugs occupy some ACh receptors, depolarization by succinylcholine is partially
prevented.
If enough depolarizing agent is
administered to develop a phase II block, a nondepolarizer will potentiate
paralysis.
Dosage
Because of the rapid onset, short
duration, and low cost of succinylcholine, many clinicians believethat it is
still a good choice for routine intubation in adults. The usual adult dose of
succinylcholine for intubation is 1–1.5 mg/kg intravenously. Doses as small as
0.5 mg/kg will often provide accept-able intubating conditions if a
defasciculating dose of a nondepolarizing agent is not used. Repeated small
boluses (10 mg) or a succinylcholine drip (1 g in 500 or 1000 mL, titrated to
effect) can be used during surgical procedures that require brief but intense paralysis
(eg, otolaryngological endos-copies). Neuromuscular function should be
fre-quently monitored with a nerve stimulator to prevent overdosing and to
watch for phase II block. The availability of intermediate-acting
nondepolar-izing muscle relaxants has reduced the popularity of succinylcholine
infusions. In the past, these infu-sions were a mainstay of ambulatory practice
in the United States.
Because succinylcholine is not lipid
soluble, it has a small volume of distribution. Per kilogram, infants and neonates
have a larger extracellular space than adults. Therefore, dosage requirements
for pediatric patients are often greater than for adults. If succinylcholine is
administered intramuscularly to
children, a dose as high as 4–5 mg/kg does not always produce complete
paralysis.
Succinylcholine should be stored under
refrig-eration (2–8°C), and should generally be used within
14 days after removal from refrigeration and exposure to room temperature.
Side Effects & Clinical Considerations
Succinylcholine is a relatively safe
drug—assuming that its many potential complications are understood and avoided.
Because of the risk of hyperkalemia, rhabdomyolysis, and cardiacarrest in
children with undiagnosed myopathies, succinylcholine is considered relatively
contraindi-cated in the routine management of children and adolescent patients.
Most clinicians have also aban-doned the routine
use of succinylcholine for adults. Succinylcholine is still useful for rapid
sequence induction and for short periods of intense paralysis because none of
the presently available nondepolar-izing muscle relaxants can match its very
rapid onset and short duration
A. Cardiovascular
Because of the resemblance of muscle
relaxants to ACh, it is not surprising that they affect cho-linergic receptors
in addition to those at the neu-romuscular junction. The entire parasympathetic
nervous system and parts of the sympathetic nervous system (sympathetic
ganglions, adrenal medulla, and sweat glands) depend on ACh as a
neurotransmitter.
Succinylcholine not only stimulates
nicotinic cholinergic receptors at the neuromuscular junc-tion, it stimulates
all ACh receptors. The cardio-vascular actions of succinylcholine are therefore
very complex. Stimulation of nicotinic receptors in parasympathetic and sympathetic
ganglia, and mus-carinic receptors in the sinoatrial node of the heart, can
increase or decrease blood pressure and heart rate. Low doses of
succinylcholine can produce neg-ative chronotropic and inotropic effects, but
higher doses usually increase heart rate and contractil-ity and elevate
circulating catecholamine levels. In most patients, the hemodynamic
consequences are inconsequential in comparison to the effects of the induction
agent and laryngoscopy.
Children are particularly susceptible to
pro-found bradycardia following administration of succinylcholine. Bradycardia
will sometimes occur in adults when a second bolus of succinylcholine is
administered approximately 3–8 min after the first dose. The dogma (based on no
real evidence) is that the succinylcholine metabolite, succinyl-monocholine,
sensitizes muscarinic cholinergic receptors in the sinoatrial node to the
second dose of succinylcholine, resulting in bradycardia. Intravenous atropine
(0.02 mg/kg in children, 0.4 mg in adults) is normally given prophylacti-cally
to children prior to the first and subsequent doses, and usually before a second dose of succi-nylcholine is given to
adults. Other arrhythmias, such as nodal bradycardia and ventricular ectopy,
have been reported.
B. Fasciculations
The onset of paralysis by
succinylcholine is usually signaled by visible motor unit contractions called
fasciculations. These can be prevented by pretreat-ment with a small dose of
nondepolarizing relax-ant. Because this pretreatment usually antagonizes a
depolarizing block, a larger dose of succinyl-choline is required (1.5 mg/kg).
Fasciculations are typically not observed in young children and elderly
patients.
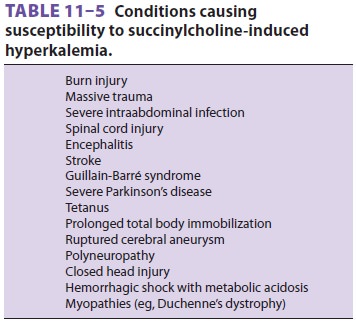
C. Hyperkalemia
Normal muscle releases enough potassium
during succinylcholine-induced depolarization to increase serum potassium by
0.5 mEq/L. Although this is usually insignificant in patients with normal
baseline potassium levels, it can be life-threatening in patients with
preexisting hyperkale-mia. The increase in potassium in patients with burn
injury, massive trauma, neurological disorders, and several other conditions (Table 11–5)
can be large and catastrophic. Hyperkalemic cardiac arrest can prove to be
quite refractory to routine cardiopul-monary resuscitation, requiring calcium,
insulin, glucose, bicarbonate, and even cardiopulmonary bypass to support the
circulation while reducing serum potassium levels.
Following denervation injuries (spinal
cord injuries, larger burns), the immature isoform of the ACh receptor may be
expressed inside and out-side the neuromuscular junction (up-regulation). These
extrajunctional receptors allow succinylcho-line to effect widespread
depolarization and exten-sive potassium release. Life-threatening potassium
release is not reliably prevented by
pretreatment with a nondepolarizer. The risk of hyperkalemia usually seems to
peak in 7–10 days following the injury, but the exact time of onset and the
duration of the risk period vary. The risk of hyperkalemia from
succinyl-choline is minimal in the first 2 days after spinal cord or burn
injury.
D. Muscle Pains
Patients who have received
succinylcholine have an increased incidence of postoperative myal-gia. The
efficacy of nondepolarizing pretreatment is controversial. Administration of
rocuronium (0.06–0.1 mg/kg) prior to succinylcholine has been reported to be
effective in preventing fasciculations and reducing postoperative myalgias. The
relation-ship between fasciculations and postoperative myal-gias is also inconsistent.
The myalgias are theorized to be due to the initial unsynchronized contraction
of muscle groups; myoglobinemia and increases in serum creatine kinase can be
detected following administration of succinylcholine. Perioperativeuse of
nonsteroidal antiinflammatory drugs may reduce the incidence and severity of
myalgias.
E. Intragastric Pressure Elevation
Abdominal wall muscle fasciculations
increase intragastric pressure, which is offset by an increase in lower
esophageal sphincter tone. Therefore, despite being much discussed, there is no
evidence that the risk of gastric reflux or pulmonary aspira-tion is increased
by succinylcholine.
F. Intraocular Pressure Elevation
Extraocular muscle differs from other
striated muscle in that it has multiple motor end-plates on each cell.
Prolonged membrane depolarization and contraction of extraocular muscles
follow-ing administration of succinylcholine transiently raise intraocular
pressure and theoretically could compromise an injured eye. However, there is no
evidence that succinylcholine leads to worsened outcome in patients with “open”
eye injuries. The elevation in intraocular pressure is not always pre-vented by
pretreatment with a nondepolarizing agent.
G. Masseter Muscle Rigidity
Succinylcholine transiently increases
muscle tone in the masseter muscles. Some difficulty may ini-tially be
encountered in opening the mouth because of incomplete relaxation of the jaw. A
marked increase in tone preventing laryngoscopy is abnor-mal and can be a
premonitory sign of malignant hyperthermia.
H. Malignant Hyperthermia
Succinylcholine is a potent triggering
agent in patients susceptible to malignant hyperthermia, a hypermetabolic
disorder of skeletal muscle . Although some of the signs and symp-toms of
neuroleptic malignant syndrome (NMS) resemble those of malignant hyperthermia,
the pathogenesis is completely different and there is no need to avoid use of
succinylcholine in patients with NMS.
I. Generalized Contractions
Patients afflicted with myotonia may
develop myoc-lonus after administration of succinylcholine.
J. Prolonged Paralysis
As discussed above, patients with
reduced levels of normal pseudocholinesterase may have a longer than normal
duration of action, whereas patients with atypical pseudocholinesterase will
experience markedly prolonged paralysis.
K. Intracranial Pressure
Succinylcholine may lead to an
activation of the electroencephalogram and slight increases in cere-bral blood
flow and intracranial pressure in some patients. Muscle fasciculations stimulate
muscle stretch receptors, which subsequently increase cerebral activity. The
increase in intracranial pres-sure can be attenuated by maintaining good airway
control and instituting hyperventilation. It can also be prevented by
pretreating with a nondepolarizing muscle relaxant and administering
intravenous lido-caine (1.5–2.0 mg/kg) 2–3 min prior to intubation. The effects
of intubation on intracranial pressure far outweigh any increase caused by
succinylcholine, and succinylcholine is NOT contraindicated for rapid sequence
induction of patients with intracra-nial mass lesions or other causes of
increased intra-cranial pressure.
L. Histamine Release
Slight histamine release may be observed
following succinylcholine in some patients.
Related Topics