Chapter: Biochemical Pharmacology : Pharmacology of nitric oxide (NO)
NO releasing drugs
NO releasing drugs
So, what
does a NO releasing drug look like? The first one there was (Figure 11.12, top)
is more widely known in an-other application, which helped Mr. Alfred Nobel
make his fortune – and still maintain his good conscience, since he believed that
this weapon would be so horrible that mankind would henceforth abstain from
warfare (a hope now held by many for nuclear weapons). The effect of
ni-troglycerine was initially noted in the form of the `Mon-day headache': The
factory workers, returning to work on Monday, experienced a strong headache8
that faded away with continuous exposure to nitroglycerine vapours dur-ing the
workweek, only to reappear the next Monday after withdrawal during the weekend.
Nitroglycerin – applied sublingually as a spray for rapid uptake and avoidance
of liver first-pass effect – is still the standard treatment of acute events of
angina pectoris, which is basically an acute, painful deterioration of coronary
artery perfusion, caused by vasoconstriction on top of atherosclerotic lesions.
One of the first patients benefiting from this treatment was – Nobel himself. I
don't know whether he actually troubled himself to get it from the pharmacy,
though.
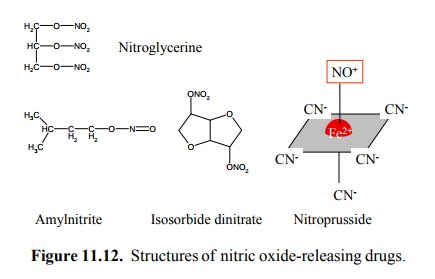
Isosorbide
dinitrate, like nitroglycerin, contains NO within nitrate groups (Figure 11.12,
bottom). Amylnitrite (which is volatile and can be inhaled) has a nitrite
instead of the nitrate group. Yet another chemistry is found with sodium
nitroprusside. While release of NO from all these drugs in vivo is rather fast
(particularly so with nitroprusside), the mechanisms are apparently different.
However, until today there is no clear picture exactly how this works. Reaction
with thiol compounds in vitro will release NO but (at least with nitrites and
nitrates) is too slow to account for the al-most instantaneous onset of drug
action in vivo. One pub-lication in 19939 described a protein
(referred to by the au-thors as an enzyme, but no physiological function was
giv en) that accelerated NO release from nitrates and was par-tially purified
from smooth muscle cell membranes. It was inhibited by thiol reagents,
suggesting the occurrence of a cysteine in the active site. While this
demonstrates the pos-sibility of protein-catalyzed release, it is unclear
whether this particular protein has a key role in it in vivo. Although this
appears interesting, I have not found any study follow-ing up on it. Knowledge
about the mode of NO release from nitrites is similarly scanty. Nitroprusside
releases NO faster with low-molecular weight thiols than nitrates do,
suggesting that enzymatic catalysis may not be necessary in vivo in this case.
Related Topics