Chapter: Medical Physiology: Membrane Physiology, Nerve, and Muscle : Contraction of Skeletal Muscle
Molecular Characteristics of the Contractile Filaments
Molecular Characteristics of the Contractile Filaments
Myosin Filament. The myosin filament is composed ofmultiple myosin molecules, each having a molecular weight of about 480,000. Figure 6–5A shows an individual molecule; Figure 6–5B shows the organization of many molecules to form a myosin filament, as well as interaction of this filament on one side with the ends of two actin filaments.
The myosin molecule (see Figure 6–5A) is composed of six polypeptide chains—two heavy chains, each with a molecular weight of about 200,000, and four lightchains with molecular weights of about 20,000 each.
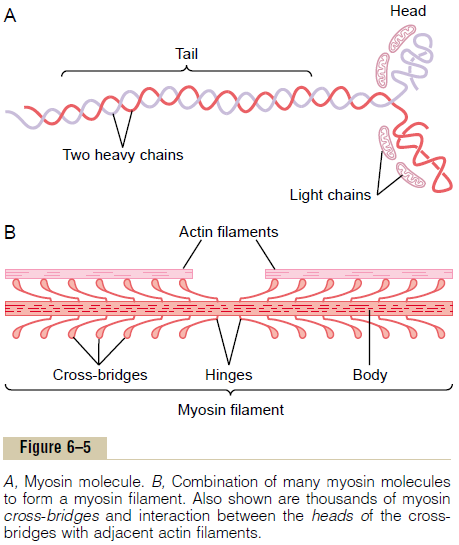
The two heavy chains wrap spirally around each other to form a double helix, which is called the tail of the myosin molecule. One end of each of these chains is folded bilaterally into a globular polypeptide structure called a myosin head. Thus, there are two free heads at one end of the double-helix myosin molecule. The four light chains are also part of the myosin head, two to each head. These light chains help control the func-tion of the head during muscle contraction.
The myosin filament is made up of 200 or more indi-vidual myosin molecules. The central portion of one of these filaments is shown in Figure 6–5B, displaying the tails of the myosin molecules bundled together to form the body of the filament, while many heads of the molecules hang outward to the sides of the body. Also, part of the body of each myosin molecule hangs to the side along with the head, thus providing an arm that extends the head outward from the body, as shown in the figure. The protruding arms and heads together are called cross-bridges. Each cross-bridge is flexible at two points called hinges—one where the arm leaves the body of the myosin filament, and the other where the head attaches to the arm. The hinged arms allow the heads either to be extended far outward from the body of the myosin filament or to be brought close to the body. The hinged heads in turn participate in the actual contraction process.
The total length of each myosin filament is uniform, almost exactly 1.6 micrometers. Note, however, that there are no cross-bridge heads in the very center of the myosin filament for a distance of about 0.2 micrometer because the hinged arms extend away from the center.
Now, to complete the picture, the myosin filament itself is twisted so that each successive pair of cross-bridges is axially displaced from the previous pair by 120 degrees. This ensures that the cross-bridges extend in all directions around the filament.
ATPase Activity of the Myosin Head. Another featureof the myosin head that is essential for muscle con-traction is that it functions as anATPase enzyme. As explained later, this property allows the head to cleave ATP and to use the energy derived from the ATP’s high-energy phosphate bond to energize the contrac-tion process.
Actin Filament. The actin filament is also complex.It is composed of three protein components: actin,tropomyosin, and troponin.
The backbone of the actin filament is a double-stranded F-actin protein molecule, represented by the two lighter-colored strands in Figure 6–6. The two strands are wound in a helix in the same manner as the myosin molecule.
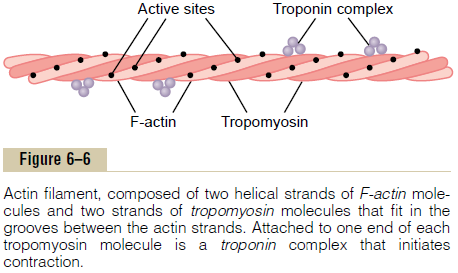
Each strand of the double F-actin helix is composed of polymerized G-actin molecules, each having a molecular weight of about 42,000. Attached to each one of the G-actin molecules is one molecule of ADP. It is believed that these ADP molecules are the active sites on the actin filaments with which the cross-bridges of the myosin filaments interact to cause muscle contraction. The active sites on the two F-actin strands of the double helix are staggered, giving one active site on the overall actin filament about every 2.7 nanometers.
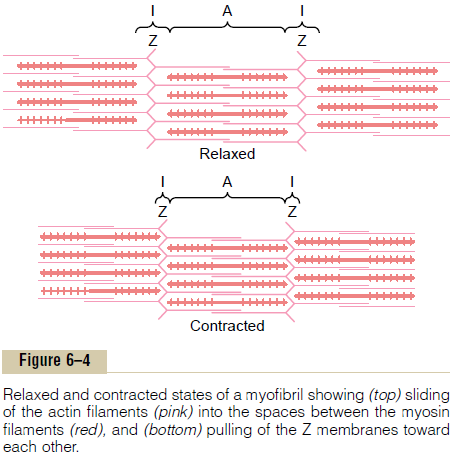
Each actin filament is about 1 micrometer long. The bases of the actin filaments are inserted strongly into the Z discs; the ends of the filaments protrude in both directions to lie in the spaces between the myosin molecules, as shown in Figure 6–4.
Tropomyosin Molecules. The actin filament also con-tains another protein, tropomyosin. Each molecule of tropomyosin has a molecular weight of 70,000 and a length of 40 nanometers. These molecules are wrapped spirally around the sides of the F-actin helix. In the resting state, the tropomyosin molecules lie on top of the active sites of the actin strands, so that attraction cannot occur between the actin and myosin filaments to cause contraction.
Troponin and Its Role in Muscle Contraction. Attachedintermittently along the sides of the tropomyosin mol-ecules are still other protein molecules called troponin. These are actually complexes of three loosely bound protein subunits, each of which plays a specific role in controlling muscle contraction. One of the subunits (troponin I) has a strong affinity for actin, another (troponin T) for tropomyosin, and a third (troponin C) for calcium ions. This complex is believed to attach the tropomyosin to the actin. The strong affinity of the troponin for calcium ions is believed to initiate the contraction process.
Interaction of One Myosin Filament, Two Actin Filaments, and Calcium Ions to Cause ContractionInhibition of the Actin Filament by the Troponin-Tropomyosin Complex; Activation by Calcium Ions. A pure actin filamentwithout the presence of the troponin-tropomyosin complex (but in the presence of magnesium ions and ATP) binds instantly and strongly with the heads of the myosin molecules. Then, if the troponin-tropomyosin complex is added to the actin filament, the binding between myosin and actin does not take place. Therefore, it is believed that the active sites on the normal actin filament of the relaxed muscle are inhibited or physically covered by the troponin-tropomyosin complex. Consequently, the sites cannot attach to the heads of the myosin filaments to cause contraction. Before contraction can take place, the inhibitory effect of the troponin-tropomyosin complex must itself be inhibited.
This brings us to the role of the calcium ions. In the presence of large amounts of calcium ions, the inhibitory effect of the troponin-tropomyosin on the actin filaments is itself inhibited. The mechanism of this is not known, but one suggestion is the follow-ing: When calcium ions combine with troponin C, each molecule of which can bind strongly with up to four calcium ions, the troponin complex supposedly under-goes a conformational change that in some way tugs on the tropomyosin molecule and moves it deeper into the groove between the two actin strands. This “uncov-ers” the active sites of the actin, thus allowing these to attract the myosin cross-bridge heads and cause con-traction to proceed. Although this is a hypothetical mechanism, it does emphasize that the normal relation between the troponin-tropomyosin complex and actin is altered by calcium ions, producing a new condition that leads to contraction.
Interaction Between the “Activated” Actin Filament and the Myosin Cross-Bridges—The “Walk-Along” Theory of Contrac-tion. As soon as the actin filament becomes activatedby the calcium ions, the heads of the cross-bridges from the myosin filaments become attracted to the active sites of the actin filament, and this, in some way, causes contraction to occur. Although the precise manner by which this interaction between the cross-bridges and the actin causes contraction is still partly theoretical, one hypothesis for which considerable evi-dence exists is the “walk-along” theory (or “ratchet”theory) of contraction.
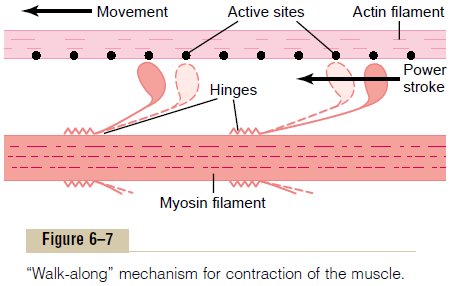
Figure 6–7 demonstrates this postulated walk-along mechanism for contraction.The figure shows the heads of two cross-bridges attaching to and disengaging from active sites of an actin filament. It is postulated that when a head attaches to an active site, this attach-ment simultaneously causes profound changes in the intramolecular forces between the head and arm of its cross-bridge. The new alignment of forces causes the head to tilt toward the arm and to drag the actin fila-ment along with it. This tilt of the head is called the power stroke. Then, immediately after tilting, the headautomatically breaks away from the active site. Next, the head returns to its extended direction. In this position, it combines with a new active site farther down along the actin filament; then the head tilts again to cause a new power stroke, and the actin filament moves another step. Thus, the heads of the cross-bridges bend back and forth and step by step walk along the actin filament, pulling the ends of two suc-cessive actin filaments toward the center of the myosin filament.
Each one of the cross-bridges is believed to operate independently of all others, each attaching and pulling in a continuous repeated cycle. Therefore, the greater the number of cross-bridges in contact with the actin filament at any given time, the greater, theoretically, the force of contraction.
ATP as the Source of Energy for Contraction—Chemical Events in the Motion of the Myosin Heads. When a muscle con-tracts, work is performed and energy is required. Large amounts of ATP are cleaved to form ADP during the contraction process; the greater the amount of work performed by the muscle, the greater the amount of ATP that is cleaved, which is called the Fenn effect. The following sequence of events is believed to be the means by which this occurs:
1. Before contraction begins, the heads of the cross-bridges bind with ATP. The ATPase activity of the myosin head immediately cleaves the ATP but leaves the cleavage products, ADP plus phosphate ion, bound to the head. In this state, the conformation of the head is such that it extends perpendicularly toward the actin filament but is not yet attached to the actin.
2. When the troponin-tropomyosin complex binds with calcium ions, active sites on the actin filament are uncovered, and the myosin heads then bind with these, as shown in Figure 6–7.
3. The bond between the head of the cross-bridge and the active site of the actin filament causes a conformational change in the head, prompting the head to tilt toward the arm of the cross-bridge. This provides the power stroke for pulling the actin filament. The energy that activates the power stroke is the energy already stored, like a “cocked” spring, by the conformational change that occurred in the head when the ATP molecule was cleaved earlier.
4. Once the head of the cross-bridge tilts, this allows release of the ADP and phosphate ion that were previously attached to the head. At the site of release of the ADP, a new molecule of ATP binds. This binding of new ATP causes detachment of the head from the actin.
5. After the head has detached from the actin, the new molecule of ATP is cleaved to begin the next cycle, leading to a new power stroke. That is, the energy again “cocks” the head back to its perpendicular condition, ready to begin the new power stroke cycle.
6. When the cocked head (with its stored energy derived from the cleaved ATP) binds with a new active site on the actin filament, it becomes uncocked and once again provides a new power stroke.
Thus, the process proceeds again and again until the actin filaments pull the Z membrane up against the ends of the myosin filaments or until the load on the muscle becomes too great for further pulling to occur.
Related Topics