Chapter: Medical Surgical Nursing: End-of-Life Care
Managing Physiologic Responses to Illness - Nursing Care of the Patient
MANAGING PHYSIOLOGIC RESPONSES TO ILLNESS
Patients
approaching the end of life experience many of the same symptoms, regardless of
their underlying disease processes. Symp-toms in terminal illness may be caused
by the disease, either di-rectly (eg, dyspnea due to chronic obstructive lung
disease) or indirectly (eg, nausea and vomiting related to pressure in the
gastric area), by the treatment for the disease, or by a coexisting disorder
that is unrelated to the disease.
The
goals of symptom management at the end of life are to completely relieve the
symptom when possible, or to decrease the symptom to a level that the patient
can tolerate when it cannot be completely relieved. Medical interventions may
be aimed at treat-ing the underlying causes of the symptoms. Pharmacologic and
nonpharmacologic methods for symptom management may be used in combination with
medical interventions to modify the physiologic causes of symptoms. For
example, some patients who develop pleural effusion secondary to metastatic
cancer may ex-perience temporary relief of the associated dyspnea following
thoracentesis, an invasive medical procedure in which fluid is drained from the
pleural space. In addition, pharmacologic man-agement with low-dose oral
morphine is very effective in relieving dyspnea, and guided relaxation may
reduce the anxiety associated with the sensation of breathlessness. As with
pain, the principles of pharmacologic symptom management are the smallest dose
of the medication to achieve the desired effect, avoidance of poly-pharmacy,
anticipation and management of medication side ef-fects, and creation of a
therapeutic regimen that is acceptable to the patient based on his or her goals
for maximizing quality of life.
As
with pain management, patients may elect to tolerate higher symptom levels in
exchange for greater independence, mobility, alertness, or other priorities.
Anticipating and planning interven-tions for symptoms that have not yet
occurred is a cornerstone of end-of-life care. Both patients and family members
cope more ef-fectively with new symptoms and exacerbations of existing
symp-toms when they know what to expect and how to manage it. Hospice programs
typically provide “emergency kits” containing ready-to-administer doses of a
variety of medications that are use-ful to treat symptoms in advanced illness.
Family members can be instructed to administer a prescribed dose from the
emergency kit, often avoiding prolonged suffering for the patient as well as
re-hospitalization for symptom management.
Pain
Pain
and suffering are among the most feared consequences of cancer (Roth &
Breitbart, 1996). Pain is a significant symptom for many cancer patients
throughout their treatment and disease course; it results both from the disease
and the modalities used to treat it. Numerous studies have indicated that
patients with ad-vanced illness, particularly cancer, experience considerable
pain (Field & Cassel, 1997; Jacox, Carr, & Payne, 1994). While the
means to relieve pain have existed for many years, the continued, pervasive
undertreatment of pain has been well documented (American Pain Society, 1999;
Jacox et al., 1994). It is estimated that as many of 70% of patients with
advanced cancer experience severe pain (Jacox et al., 1994; World Health
Organization, 1990). The impact of poorly managed pain on patients’
psychological, emotional, social, and financial well-being has attracted
con-siderable research interest, but practice has been slow to change (Spross,
1992).
Patients
who have an established regimen of analgesics should continue to receive those
medications as they approach the end of life. Inability to communicate pain
should not be equated with the absence of pain. While most pain can be managed
effectively using the oral route, as the end of life nears patients may be less
able to swallow oral medications due to somnolence or nausea. Patients who have
been receiving opioids should continue to re-ceive equianalgesic doses via the
rectal or sublingual routes. Con-centrated morphine solution can be very
effectively delivered by the sublingual route, as the small liquid volume is
well tolerated even when the patient cannot swallow. As long as the patient
con-tinues to receive opioids, a regimen to combat constipation must be
implemented. If the patient cannot swallow laxatives or stool softeners, rectal
suppositories or enemas may be necessary.
The
nurse should teach the family about continuation of com-fort measures as the
patient approaches the end of life, how to ad-minister analgesics via alternate
routes, and how to assess for pain when the patient cannot verbally report pain
intensity. Because the analgesics administered orally or rectally are
short-acting, typically scheduled as frequently as every 3 to 4 hours around
the clock, there is always a strong possibility that the patient approaching
the end of life will die in close proximity to the time of analgesic
ad-ministration. If the patient is at home, family members adminis-tering
analgesics need to be prepared for this possibility. They will need reassurance
that they did not “cause” the death of the patient by administering a dose of
analgesic medication (see Chart 13-3).
Dyspnea
Dyspnea
is an uncomfortable awareness of breathing that is common in patients
approaching the end of life (Brant, 1998). Dyspnea is a highly subjective
symptom that often is not associ-ated with visible signs of distress, such as
tachypnea, diaphoresis, or cyanosis. Patients with primary lung tumors, lung
metastases, pleural effusion, and restrictive lung disease may experience
sig-nificant dyspnea. Although the underlying cause of the dyspnea can be
identified and treated in some cases, the burdens of addi-tional diagnostic evaluation
and treatment aimed at the physio-logical problem may outweigh the benefits.
The treatment of dyspnea varies depending on the patient’s general physical
con-dition and imminence of death. For example, a blood transfusion may provide
temporary symptom relief for the anemic patient earlier in the disease process;
however, as the patient approaches the end of life the benefits are typically
short-lived or absent.
NURSING ASSESSMENT AND INTERVENTION
As
is true in pain assessment and management, the patient’s re-port of dyspnea
must be believed. Also like the experience of physical pain, the meaning of the
dyspnea to the patient may in-crease his or her suffering. For example, the
patient may interpret increasing dyspnea as a sign that death is approaching.
For some patients, sensations of breathlessness may invoke frightening im-ages
of drowning or suffocation, and the resulting cycle of fear and anxiety may
create even greater sensations of breathlessness. Therefore, the nurse should
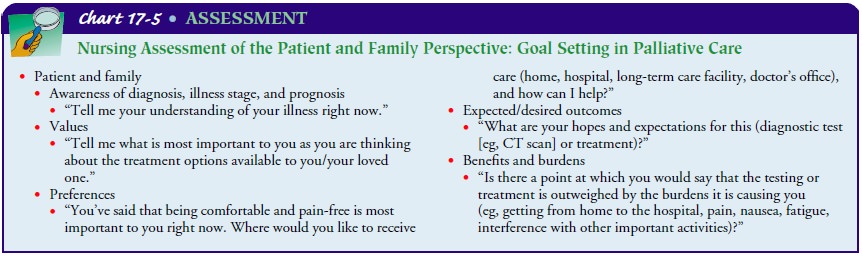
conduct a careful assessment of the psychosocial and spiritual components of
the symptom (see Chart 17-5). Physical assessment parameters include:
·
Symptom intensity, distress, and
interference with activities (scale of 0 to 10)
·
Auscultation of lung sounds
·
Assessment of fluid balance
·
Measurement of dependent edema
(circumference of lower extremities)
·
Measurement of abdominal girth
·
Temperature
·
Skin color
·
Sputum quantity and character
·
Cough
To
determine the intensity of the symptom and its interference with daily activities,
patients can be asked to self-report using a scale of 0 to 10, where 0 is no
dyspnea and 10 is the worst imag-inable dyspnea. Measurement of the patient’s
baseline before treatment and subsequent measures during exacerbation of the
symptom, periodically during treatment, and whenever the treat-ment plan
changes will provide ongoing objective evidence for the efficacy of the
treatment plan. In addition, physical assessment findings may assist in
locating the source of the dyspnea and se-lecting nursing interventions to
relieve the symptom. The com-ponents of the assessment will change as the
patient’s condition changes. For example, when the patient who has been on
daily weights can no longer get out of bed, the goal of comfort may out-weigh
the benefit of continued weights. Like other symptoms at the end of life,
dyspnea can be managed effectively in the absence of assessment and diagnostic
data (ie, arterial blood gases) that are standard when the patient’s illness or
symptom is reversible.
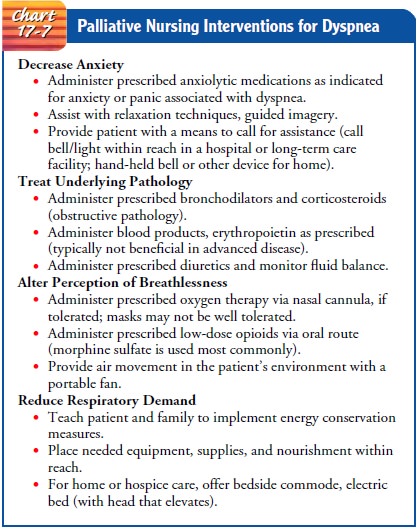
Nursing
management of dyspnea at the end of life is directed toward administering
medical treatment for the underlying pathol-ogy, monitoring the patient’s
response to treatment, assisting the patient and family to manage anxiety
(which exacerbates dyspnea),altering the perception of the symptom, and
conserving energy (Chart 17-7). Pharmacologic intervention is aimed at
modifying lung physiology and improving performance as well as altering the
perception of the symptom. Bronchodilators and cortico-steroids are examples of
medications used to treat underlying ob-structive pathology, thereby improving
overall lung function. Low doses of opioids are very effective in relieving
dyspnea, although the mechanism of relief is not entirely clear. Although
dyspnea in terminal illness is typically not associated with dimin-ished blood
oxygen saturation, low-flow oxygen often provides psychological comfort to the
patient and the family, particularly in the home setting.
As
discussed above, dyspnea may be exacerbated by anxiety, and anxiety may trigger
episodes of dyspnea, setting off a respi-ratory crisis in which patient and
family may panic. For patients receiving care at home, patient and family
instruction should in-clude anticipation and management of crisis situations and
a clearly communicated emergency plan. Patients and families should be
instructed about medication administration, condition changes that should be
reported to the physician and nurse, and strategies for coping with diminished
reserves and increasing symptomatology as the disease progresses. The patient
and fam-ily need reassurance that the symptom can be effectively managed at
home without the need for activation of the emergency med-ical services or
hospitalization and that a nurse will be available at all times via telephone
or to conduct a visit.
Nutrition and Hydration at the End of Life
ANOREXIA
Anorexia and cachexia are common problems in the seriously ill. The profound changes in the patient’s appearance and his or her concomitant lack of interest in the socially important rituals of mealtime are particularly disturbing to families.

The approach to the problem varies depending on the
patient’s stage of illness, level of disability associated with the illness,
and desires. The anorexia-cachexia syndrome is characterized by disturbances in
carbohydrate, protein, and fat metabolism, endocrine dysfunc-tion, and anemia.
The syndrome results in severe asthenia (loss of energy). Although causes of
anorexia may be controlled for a pe-riod of time, progressive anorexia is an
expected and natural part of the dying process. Anorexia may be related to or
exacerbated by situational variables (eg, the ability to have meals with the
fam-ily versus eating alone in the “sick room”), progression of the dis-ease,
treatment for the disease, or psychological distress. The patient and family
should be instructed in strategies to manage the variables associated with
anorexia. Table 17-2 summarizes nursing measures and patient and family
teaching for managing anorexia.
USE OF PHARMACOLOGIC AGENTS TO STIMULATE APPETITE IN THE TERMINALLY ILL
A
number of pharmacologic agents are commonly used to stim-ulate appetite in
anorectic patients. Commonly used medications for appetite stimulation include
dexamethasone (Decadron), cypro-heptadine (Periactin), megestrol acetate
(Megace), and dronabi-nol (Marinol). Dexamethasone initially increases appetite
and may provide short-term weight gain in some patients. However, therapy may
need to be discontinued in the patient with a longer life expectancy, as after
3 to 4 weeks corticosteroids interfere with the synthesis of muscle protein.
Cyproheptadine may be used when corticosteroids are contraindicated, such as
when the pa-tient is diabetic. It promotes mild appetite increase but no
ap-preciable weight gain. Megestrol acetate produces temporary weight gain of
primarily fatty tissue, with little effect on protein balance. Because of the
time required to see any effect from this agent, therapy should not be
initiated if life expectancy is less than 30 days. Finally, dronabinol is a
psychoactive compound found in cannabis that may be helpful in reducing nausea
and vomiting, appetite loss, pain, and anxiety, thereby improving intake in
some patients. However, dronabinol is not as effective as the other agents for
appetite stimulation in most patients. Although the use of these agents may
cause temporary weight gain, their use is not associated with an increase in
lean body mass in the terminally ill. Therapy should be tapered or discontinued
after 4 to 8 weeks if there is no response (Wrede-Seaman, 1999).
CACHEXIA
Cachexia
refers to severe muscle wasting and weight loss associated with illness.
Although anorexia may exacerbate cachexia, it is not the primary cause. Cachexia
is associated with changes in metab-olism that include hypertriglyceridemia,
lipolysis, and accelerated protein turnover, leading to depletion of fat and
protein stores (Plata-Salaman, 1997). However, the pathophysiology of cachexia
in terminal illness is not well understood. In terminal illness, the severity
of tissue wasting is greater than would be expected from reduced food intake
alone, and typically increasing appetite or food intake does not reverse
cachexia in the terminally ill.
Anorexia and cachexia differ from starvation (simple food deprivation) in several important ways. Appetite is lost early in the process, the body becomes catabolic in a dysfunctional way, and supplementation by gastric feeding (tube feeding) or par-enteral nutrition in advanced disease does not replenish lost lean body mass. At one time it was believed that cancer patients with rapidly growing tumors developed cachexia because the tumor created an excessive nutritional demand and diverted nutrients from the rest of the body.
Recent research links cy-tokines produced by the body in response to
a tumor to a com-plex inflammatory-immune response present in patients whose
tumors have metastasized, leading to anorexia, weight loss, and altered
metabolism. An increase in cytokines occurs not only in cancer but also in AIDS
and many other chronic diseases (Plata-Salaman, 1997).
ARTIFICIAL NUTRITION AND HYDRATION IN TERMINAL ILLNESS
Along
with breathing, eating and drinking are essential to survival throughout one’s
lifetime. As patients near the end of life, their bodies’ nutritional needs
change, their desire for food and fluid may diminish, and they may no longer be
able to use, eliminate, or store nutrients and fluids adequately. Eating,
feeding, and shar-ing meals are important social activities in families and
commu-nities, and food preparation and enjoyment are linked to happy memories,
strong emotions, and hopes for survival. For the patient with serious illness,
food preparation and mealtimes often become battlegrounds where well-meaning
family members argue, plead, and cajole to encourage the ill person to eat. It
is not unusual for seriously ill patients to lose their appetites entirely, to
develop strong aversions for foods they have enjoyed in the past, or to crave a
particular food to the exclusion of all other foods.
Although
nutritional supplementation may be an important part of the treatment plan in
early or chronic illness, unintended weight loss and dehydration are expected
sequelae of progressive illness. As illness progresses, patients, families, and
clinicians may believe that without artificial nutrition and hydration, the
termi-nally ill patient will “starve,” causing profound suffering and has-tened
death. However, starvation should not be viewed as the failure to implant tubes
for nutritional supplementation or hy-dration of terminally ill patients with
irreversible progression of disease. Studies have demonstrated that terminally
ill patients who were hydrated had neither improved biochemical parame-ters nor
improved states of consciousness (Waller, Hershkowitz Adunsky, 1994).
Similarly, survival was not increased when terminally ill patients with
advanced dementia received enteral feeding (Meier, Ahronheim, Morris et al.,
2001). Further, in pa-tients who are close to death there are beneficial
effects to with-holding or withdrawing artificial nutrition and hydration, such
as decreased urine output and incontinence, decreased gastric flu-ids and
emesis, decreased pulmonary secretions and respiratory distress, and decreased
edema and pressure discomfort (Zerwekh, 1987).
As
the patient approaches the end of life, families and health care providers
should offer the patient what he or she desires and can most easily tolerate.
Nurses should instruct the family how to separate feeding from caring by
demonstrating love, sharing, and caring by being with the loved one in other
ways. Preoccupation with appetite, feeding, and weight loss diverts energy and
time that the patient and family could use in other meaningful activi-ties. The
following are tips to promote nutrition for the termi-nally ill patient:
·
Offer small portions of favorite
foods.
·
Do not be overly concerned about a
“balanced” diet.
·
Cool foods may be better tolerated
than hot foods.
·
Offer cheese, eggs, peanut butter,
mild fish, chicken, or turkey. Meat (especially beef) may taste bitter and
unpleasant.
·
Add milkshakes, “Instant Breakfast”
drinks, or other liquid supplements.
·
Add dry milk powder to milkshakes
and cream soups to in-crease protein and calorie content.
·
Place nutritious foods at the
bedside (fruit juices, milk-shakes in insulated drink containers with straws).
·
Schedule meals when family members
can be present to provide company and stimulation.
·
Avoid arguments at mealtime.
·
Assist the patient to maintain a
schedule of oral care. Rinse the mouth after each meal or snack. Avoid
mouthwashes that contain alcohol. Use a soft toothbrush. Treat ulcers or
lesions. Make sure dentures fit well.
·
Treat pain and other symptoms.
·
Offer ice chips made from frozen
fruit juices.
·
Allow the patient to refuse foods
and fluids.
Delirium
Many
patients may remain alert, arousable, and able to commu-nicate until very close
to death. Others may sleep for long inter-vals and awaken only intermittently,
with eventual somnolence until death. Delirium refers to concurrent
disturbances in level of consciousness, psychomotor behavior, memory, thinking,
atten-tion, and sleep-wake cycle (Brant, 1998). In some patients, a pe-riod of
agitated delirium may precede death, sometimes causing families to be hopeful
that the suddenly active patient may be get-ting better. Confusion may be
related to underlying, treatable conditions such as medication side effects or
interactions, pain or discomfort, hypoxia or dyspnea, a full bladder or
impacted stool. In patients with cancer, confusion may be secondary to brain
metastases. Delirium may also be related to metabolic changes, infection, and
organ failure.
The
patient with delirium may become hypoactive or hyper-active, restless,
irritable, and fearful. Sleep deprivation and hal-lucinations may occur. If
treatment of the underlying factors contributing to these symptoms bring no
relief, a combination of pharmacologic intervention with neuroleptics or benzodiazepines
may be effective in decreasing distressing symptoms. Haloperidol (Haldol) may
reduce hallucinations and agitation. Benzodiazepines (eg, lorazepam [Ativan])
can reduce anxiety but will not clear the sensorium and may contribute to
worsening cognitive impair-ment if used alone.
Nursing
interventions are aimed at identifying the underlying causes of delirium,
acknowledging the family’s distress over its oc-currence, reassuring them about
what is normal, teaching the family how to interact with and ensure safety for
the patient with delirium, and monitoring the effects of medications used to
treat severe agitation, paranoia, or fear. Confusion may mask the patient’s
unmet spiritual needs and fears about dying. Spiritual inter-vention, music
therapy, gentle massage, and therapeutic touch may provide some relief.
Reducing environmental stimuli, avoiding harsh lighting or very dim lighting
(which may produce disturbing shadows), the presence of familiar faces, and
gentle reorientation and reassurance are also helpful.
Depression
Clinical
depression should not be accepted as an inevitable con-sequence of dying, nor
should it be confused with sadness and an-ticipatory grieving, which are normal
reactions to the losses associated with impending death. Emotional and
spiritual sup-port and control of disturbing physical symptoms are appro-priate
interventions for situational depression associated with terminal illness. The
psychological sequelae of cancer pain have been linked to suicidal thought and
less frequently to carrying out a planned suicide (Ripamonti, Filiberti, Totis
et al., 1999). Can-cer patients with advanced disease are especially vulnerable
to delirium, depression, suicidal ideation, and severe anxiety (Roth
Breitbart,
1996). Higher levels of debilitation predict higher levels of pain and
depressive symptoms, and the presence of pain doubles the likelihood of
developing major psychiatric complica-tions of illness (Roth & Breitbart,
1996). Patients and their fam-ilies must be given space and time to experience
sadness and to grieve, but patients should not have to endure untreated
depres-sion at the end of their lives. An effective combined approach to
clinical depression includes relief of physical symptoms, attention to
emotional and spiritual distress, and pharmacologic inter-vention with
psychostimulants, selective serotonin reuptake in-hibitors (SSRIs), and
tricyclic antidepressants (Block, 2000).
Related Topics