Chapter: Clinical Anesthesiology: Anesthetic Management: Respiratory Physiology& Anesthesia
Lung Mechanics
LUNG MECHANICS
The movement of the lungs is passive and
deter-mined by the impedance of the respiratory system, which can be divided
into the elastic resistance of tissues and the gas–liquid interface and the
nonelas-tic resistance to gas flow. Elastic resistance governs lung volume and
the associated pressures under static conditions (no gas flow). Resistance to
gas flow relates to frictional resistance to airflow and tissue deformation.
The work necessary to overcome elas-tic resistance is stored as potential
energy, but the work necessary to overcome nonelastic resistance is lost as
heat.
1. Elastic Resistance
Both the lungs and the chest have
elastic proper-ties. The chest has a tendency to expand outward, whereas the
lungs have a tendency to collapse. When the chest is exposed to atmospheric
pressure (open pneumothorax), it usually expands about 1 L in adults. In
contrast, when the lung is exposed to atmospheric pressure, it collapses
completely and all the gas within it is expelled. The recoil properties of the
chest are due to structural components that resist deformation and chest wall
muscle tone. The elastic recoil of the lungs is due to their high content of
elastin fibers, and, even more important, the sur-face tension forces acting at
the air–fluid interface in alveoli.
Surface Tension Forces
The gas–fluid interface lining the alveoli causes them to behave as bubbles. Surface tension forces tend to reduce the area of the interface and favor alveolar collapse. Laplace’s law can be used to quantify these forces:
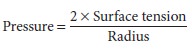
The pressure derived from the equation is that within the alveolus. Alveolar collapse is therefore directly proportional to surface tension. Fortunately,in contrast to a bubble, pulmonary surfactant decreases alveolar surface tension. Moreover, the ability of the surfactant to lower surface tension is directly proportional to its concentration within the alveolus, resulting in lower intraalveolar pressure in smaller alveoli. As alveoli become smaller, the sur-factant within becomes more concentrated, and sur-face tension is more effectively reduced. Conversely, when alveoli are overdistended, surfactant becomes less concentrated, and surface tension increases. The net effect is to stabilize alveoli; small alveoli are pre-vented from getting smaller, whereas large alveoli are prevented from getting larger.
Compliance
Elastic
recoil is usually measured in terms of com-pliance (C), which is defined as the
change in vol-ume divided by the change in distending pressure. Compliance
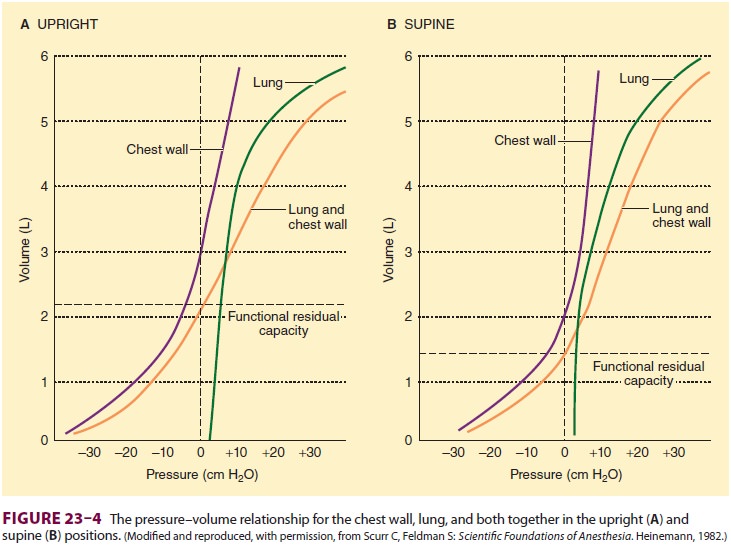
measurements can be obtained for either the chest, the lung, or both together (Figure 23–4).
In
the supine position, chest wall compliance (Cw) is reduced because of the
weight of the abdominal contents against the diaphragm. Measurements are
usually obtained under static conditions, (ie, at equilibrium). (Dynamic lung
compliance [Cdyn,l], which is measured during rhythmic breathing, is also
dependent on airway resistance.) Lung
compli-ance (Cl) is defined as
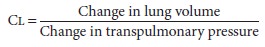
Cl
is normally 150–200 mL/cm H2O. A variety of factors, including lung
volume, pulmonary blood volume, extravascular lung water, and pathological
processes (eg, inflammation and fibrosis) affect Cl
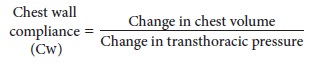
where
transthoracic pressure equals atmospheric pressure minus intrapleural
pressure.Normal chest wall compliance is 200 mL/ cm H2O. Total compliance
(lung and chest wall together) is 100 mL/cm H2O and is expressed by
the following equation:
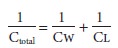
2. Lung Volumes
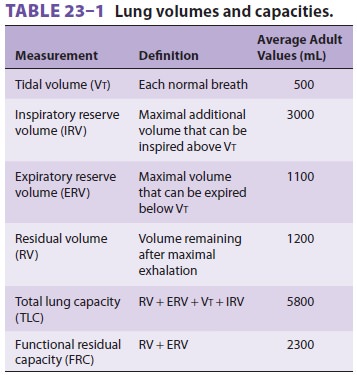
Lung volumes are important parameters in
respira-tory physiology and clinical practice ( Table 23–1 and Figure 23–5).
The sum of all of the named lung volumes equals the maximum to which the lung
can be inflated. Lung capacities are clinically useful measurements that
represent a combination of two or more volumes.
Functional Residual Capacity
The lung volume at the end of a normal exha-lation is called functional residual capacity(FRC). At this volume, the inward elastic recoil of the lung approximates the outward elastic recoil of the chest (including resting diaphragmatic tone). Thus, the elastic properties of both chest and lung define the point from which normal breathing takes place. Functional residual capacity can be measured by nitrogen washout or helium washin technique or by body plethysmography. Factors known to alter the FRC include the following:
Body
habitus: FRC is directly
proportionalto height. Obesity, however, can markedly decrease FRC (primarily
as a result of reduced chest compliance).
Sex:
FRC is reduced by about
10% in femalescompared with males.
Posture:
FRC decreases as a
patient is movedfrom an upright to a supine or prone position. This is the
result of reduced chest compliance as the abdominal contents push up against
the diaphragm. The greatest change occurs between 0° and 60°
of inclination. No further decrease is observed with a head-down position of up
to 30°.
Lung
disease: Decreased
compliance ofthe lung, chest, or both is characteristic of restrictive
pulmonary disorders all of which are associated with a low FRC.
Diaphragmatic
tone: This
normallycontributes to FRC.
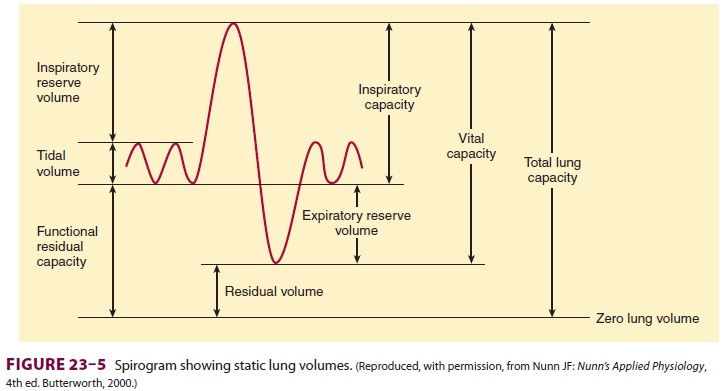
Closing Capacity
As described above (see the section on
Functional Respiratory Anatomy), small airways lacking carti-laginous support
depend on radial traction caused by the elastic recoil of surrounding tissue to
keep them open; patency of these airways, particularly in basal areas of the
lung, is highly dependent on lung volume. The volume at which these airways
begin to close in dependent areas of the lung is called the closing capacity. At lower lung
volumes, alveoli independent areas continue to be perfused but are no longer
ventilated; intrapulmonary shunting
of deoxygenated blood promotes hypoxemia .
Closing capacity is usually measured
using a tracer gas (xenon-133), which is inhaled near resid-ual volume and then
exhaled from total lung capacity.Closing capacity is normally well below FRC (Figure 23–6),
but rises steadily with age (Figure 23–7). This increase is probably
responsible for the normal age-related decline in arterial O 2 ten-sion. At an average age of 44 years, closing
capacity
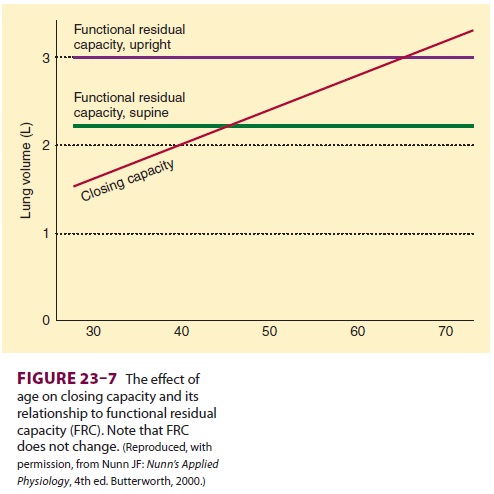
equals FRC in the supine position; by
age 66, closing capacity equals or exceeds FRC in the upright posi-tion in most
individuals. Unlike FRC, closing capac-ity is unaffected by posture.
Vital Capacity
Vital capacity (VC) is the maximum
volume of gas that can be exhaled following maximal inspiration. In addition to
body habitus, VC is also dependent on respiratory muscle strength and
chest–lung compli-ance. Normal VC is about 60–70 mL/kg.
3. Nonelastic Resistances
Airway Resistance to Gas Flow
Gas flow in the lung is a mixture of
laminar and turbulent flow. Laminar flow can be thought of as consisting of
concentric cylinders of gas flowing at dif-ferent velocities; velocity is
highest in the center and decreases toward the periphery. During laminar flow,
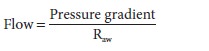
where Raw
is airway resistance.
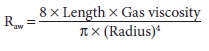
Turbulent flow is characterized by
random movement of the gas molecules down the air pas-sages. Mathematical
description of turbulent flow is considerably more complex:
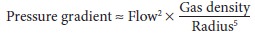
Resistance is not constant but increases
in pro-portion to gas flow. Moreover, resistance is directly proportional to
gas density and inversely proportional to the fifth power of the radius. As a
result, turbulent gas flow is extremely sensitive to airway caliber.
Turbulence generally occurs at high gas
flows, at sharp angles or branching points, and in response to abrupt changes
in airway diameter. Whether turbu-lent or laminar flow occurs can be predicted
by the Reynolds number, which results from the following equation:
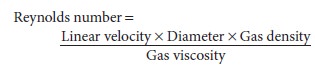
A low Reynolds number (<1000) is associated with laminar flow, whereas a
high value (>1500) produces turbulent flow. Laminar
flow normally occurs only distal to small bronchioles (<1 mm). Flow in larger airways is probably turbulent.
Of the
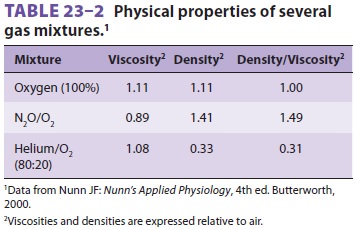
gases used clinically, only helium has a
significantly lower density-to-viscosity ratio, making it useful clinically
during severe turbulent flow (as caused by upper airway obstruction). A
helium–O2 mixture not only is less likely to
cause turbulent flow but also reduces airway resistance when turbulent flow is
present (Table
23–2).
Normal total airway resistance is about
0.5–2 cm H2O/L/sec, with the largest contribution
coming from medium-sized bronchi (before the seventh generation). Resistance in
large bronchi is low because of their large diameters, whereas resistance in
small bronchi is low because of their large total cross-sectional area. The
most impor-tant causes of increased airway resistance include bronchospasm,
secretions, and mucosal edema as well as volume-related and flow-related airway
collapse.
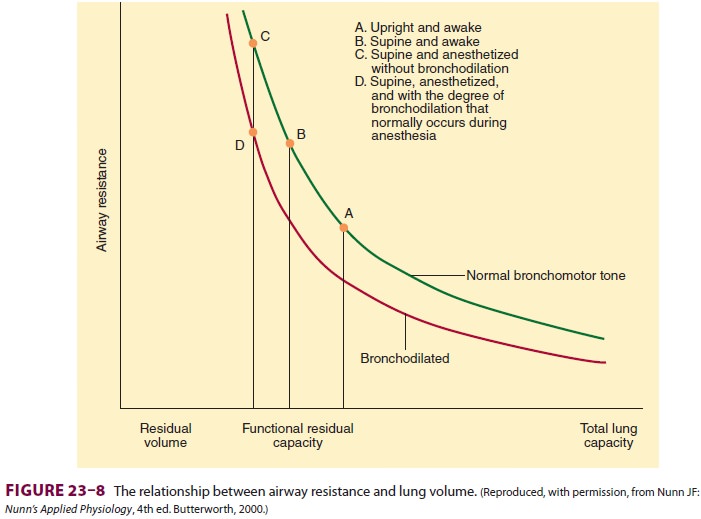
A. Volume-Related Airway Collapse
At low lung volumes, loss of radial
traction increases the contribution of small airways to total resistance;
airway resistance becomes inversely proportional to lung volume ( Figure 23–8).
Increasing lung volume up to normal with positive end-expiratory pressure
(PEEP) can reduce airway resistance.
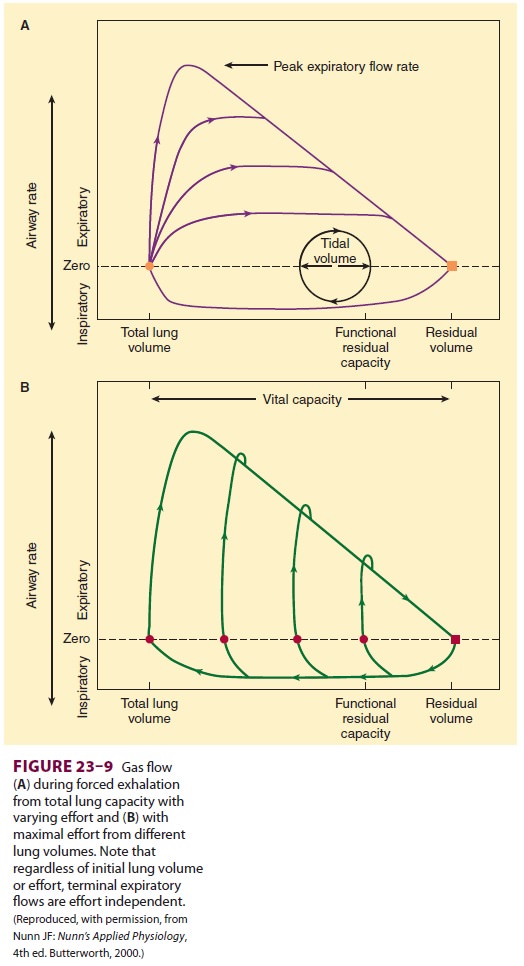
B. Flow-Related Airway Collapse
During forced exhalation, reversal of
the normal transmural airway pressure can cause collapse of these airways
(dynamic airway compression). Two contributing factors are responsible:
generation of a positive pleural pressure and a large pressure drop across
intrathoracic airways as a result of increased airway resistance. The latter is
in turn due to high (turbulent) gas flow
and the reduced lung volume. The terminal portion of the flow/volume curve is
therefore considered to be effort independent (Figure 23–9).
The point along the airways where
dynamic compression occurs is called the equal pressure point. It is normally
beyond the eleventh to thirteenth gen-eration of bronchioles where
cartilaginous support is
absent (see above). The equal pressure
point moves toward smaller airways as lung volume decreases. Emphysema or
asthma predisposes patients to dynamic airway compression. Emphysema destroys
the elastic tissues that normally support smaller air-ways. In patients with
asthma, bronchoconstriction and mucosal edema intensify airway collapse and
promote reversal of transmural pressure gradients across airways. Patients may
terminate exhalation prematurely or purse their lips to increase expiratory
resistance at the mouth. Premature termination of exhalation may increase FRC
above normal, result-ing in air trapping and auto-PEEP.
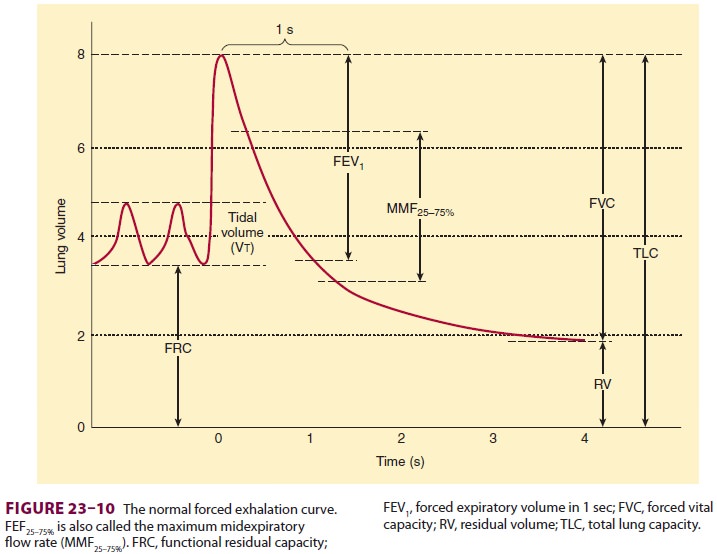
C. Forced Vital Capacity
Measuring vital capacity as an
exhalation that is as forceful and rapid as possible (Figure 23–10) provides important
information about airway resis-tance. The ratio of the forced expiratory volume
in the first second of exhalation (FEV1)
to the total forced vital capacity (FVC) is proportional to the degree of
airway obstruction. Normally, FEV1/FVCis ≥80%. Whereas both FEV1
and FVC are effort dependent, forced midexpiratory flow
(FEF25–75%) is more effort independent and may be a more
reliable measurement of obstruction.
Tissue Resistance
Th is component of nonelastic resistance is gener-ally underestimated and often overlooked, but may account for up to half of total airway resistance. It seems to be primarily due to viscoelastic (frictional) resistance of tissues to gas flow.
4. Work of Breathing
Because expiration is normally entirely
passive, both the inspiratory and the expiratory work of breathing is performed
by the inspiratory muscles (primarily the diaphragm). Three factors must be
overcome during ventilation: the elastic recoil of the chest and lung, frictional
resistance to gas flow in the airways, and tissue frictional resistance.
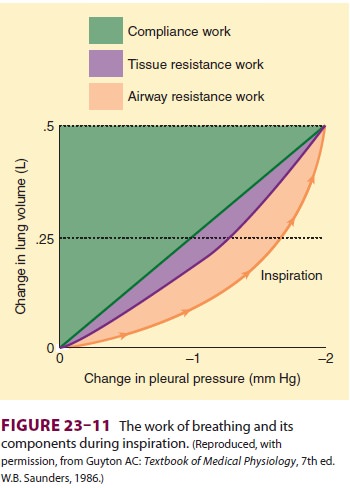
Respiratory work can be expressed as the
prod-uct of volume and pressure ( Figure 23–11). During inhalation, both
inspiratory airway resistance and pulmonary elastic recoil must be overcome;
nearly 50% of the energy expended is stored pulmonary elastic recoil. During
exhalation, the stored potential energy is released and overcomes expiratory
airway resistance. Increases in either inspiratory or expira-tory resistance
are compensated by increased inspi-ratory muscle effort. When expiratory
resistance increases, the normal compensatory response is to increase lung
volume such that Vt breathing occurs at an abnormally high FRC. The greater
elastic recoilenergy stored at a higher lung volume overcomes the added
expiratory resistance. Excessive amounts of expiratory resistance also activate
expiratory mus-cles (see above).Respiratory muscles normally account for only
2% to 3% of O2 consumption but operate at about 10%
efficiency. Ninety percent of the work is dissi-pated as heat (due to elastic
and airflow resistance). In pathological conditions that increase the load on
the diaphragm, muscle efficiency usually progressively decreases, and
contraction may become uncoordi-nated with increasing ventilatory effort;
moreover, a point may be reached whereby any increase in O2 uptake (because of augmented ventilation) is
con-sumed by the respiratory muscles themselves.
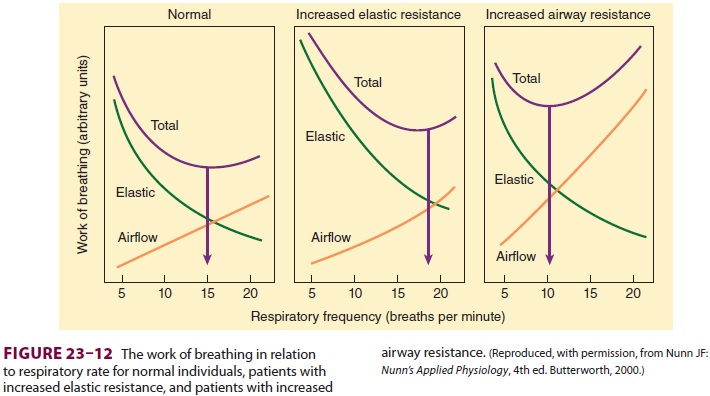
The work required to overcome elastic
resis-tance increases as Vt increases, whereas the work required to overcome
airflow resistance increases as respiratory rate (and, necessarily, expiratory
flow) increases. Faced with either condition, patients minimize the work of
breathing by altering the respiratory rate and Vt (Figure 23–12). Patientswith reduced compliance tend to
have rapid, shal-low breaths, whereas those with increased airflow resistance
have a slow, deep breathing pattern.
5. Effects of Anesthesia on Pulmonary Mechanics
The effects of anesthesia on breathing
are complex and relate to changes both in position and anesthetic agent.
Effects on Lung Volumes & Compliance
Changes in lung mechanics due to general
anes-thesia occur shortly after induction. The supineposition reduces the FRC
by 0.8–1.0 L, and induc-tion of general anesthesia further reduces the FRC by
0.4–0.5 L. FRC reduction is a consequence of alveolar collapse and compression
atelectasis due to loss of inspiratory muscle tone, change in chest wall
rigidity, and upward shift of the diaphragm. The mechanisms may be more
complex; for example, only the depen-dent (dorsal) part of the diaphragm in the
supine position moves cephalad. Other factors are likely due to a change in
intrathoracic volume secondary to increased blood volume in the lung and
changes in
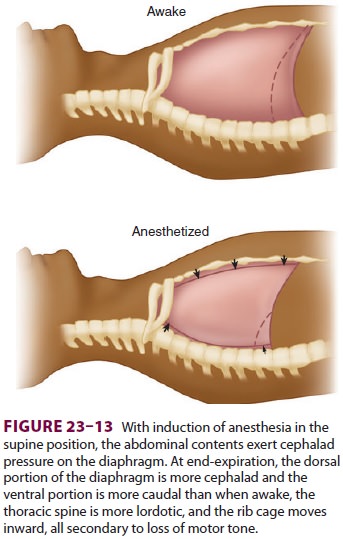
chest wall shape (Figure 23–13). The higher
position of the dorsal diaphragm and changes in the thoracic cavity itself
decrease lung volumes. This decrease in FRC is not related to anesthetic depth
and may per-sist for several hours or days after anesthesia. Steep head-down
(Trendelenburg) position (>30°) may reduce FRC even further as intrathoracic blood
vol-ume increases. In contrast, induction of anesthesia in the sitting position
seems to have little effect on FRC. Muscle paralysis does not seem to change
FRC sig-nificantly when the patient is already anesthetized.
The effects of anesthesia on closing
capacity are more variable. Both FRC and closing capacity, however, are
generally reduced to the same extent under anesthesia. Thus, the risk of
increased intra-pulmonary shunting under anesthesia is similar to that in the
conscious state; it is greatest in the elderly, in obese patients, and in those
with underlying pul-monary disease.
Effects on Airway Resistance
The reduction in FRC associated with general anes-thesia would be expected to increase airway resis-tance. Increases in airway resistance are not usually observed, however, because of the bronchodilating properties of the volatile inhalation anesthetics.
Increased airway resistance is more
commonly due to pathological factors (posterior displacement of the tongue;
laryngospasm; bronchoconstriction; or secretions, blood, or tumor in the
airway) or equip-ment problems (small tracheal tubes or connectors, malfunction
of valves, or obstruction of the breath-ing circuit).
Effects on the Work of Breathing
Increases in the work of breathing under
anesthesia are most often secondary to reduced lung and chest wall compliance,
and, less commonly, increases in airway resistance (see above). The problems of
increased work of breathing are usually circum-vented by controlled mechanical
ventilation.
Effects on the Respiratory Pattern
Regardless of the agent used, light
anesthesia often results in irregular breathing patterns; breath hold-ing is
common. Breaths become regular with deeper levels of anesthesia. Inhalation
agents generally pro-duce rapid, shallow breaths, whereas nitrous–opioid
techniques result in slow, deep breaths.
Related Topics