Chapter: Clinical Anesthesiology: Anesthetic Management: Respiratory Physiology& Anesthesia
Control of Breathing
CONTROL OF BREATHING
Spontaneous
ventilation is the result of rhythmic neural activity in respiratory centers
within the brainstem. This activity regulates respiratory mus-cles to maintain
normal tensions of O 2 and CO 2 in the body. The basic
neuronal activity is modified by inputs from other areas in the brain,
volitional and autonomic, as well as
various central and peripheral receptors (sensors).
1. Central Respiratory Centers
The basic breathing rhythm originates in
the medulla. Two medullary groups of neurons are gen-erally recognized: a
dorsal respiratory group, which is primarily active during inspiration, and a
ventral respiratory group, which is active during expiration. The close
association of the dorsal respiratory group of neurons with the tractus
solitarius may explain reflex changes in breathing from vagal or
glossopha-ryngeal nerve stimulation.
Two pontine areas influence the dorsal
(inspi-ratory) medullary center. A lower pontine (apneus-tic) center is
excitatory, whereas an upper pontine (pneumotaxic) center is inhibitory. The
pontine cen-ters appear to fine-tune respiratory rate and rhythm.
2. Central Sensors
The
most important of these sensors are chemore-ceptors that respond to changes in
hydrogen ion concentration. Central chemoreceptors are thought to lie on the anterolateral
surface ofthe medulla and respond primarily to changes in cerebrospinal fluid
(CSF) [H +]. This mechanism is
effective in regulating Paco2, because the blood– brain barrier is
permeable to dissolved CO2, but not to bicarbonate ions. Acute changes
in Paco 2, but not in arterial [HCO 3–], are
reflected in CSF; thus, a change in CO2 must result in a change in
[H+]:
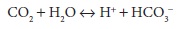
Over
the course of a few days, CSF [HCO3–] can compensate to
match any change in arterial [HCO 3–].Increases in Paco2
elevate CSF hydrogen ion concentration and activate the chemoreceptors.
Secondary stimulation of the adjacent respiratory medullary centers increases
alveolar ventilation (Figure23–25) and reduces Paco2 back
to normal. Conversely, decreases in CSF hydrogen ion con-centration secondary
to reductions in Paco2 reduce alveolar ventilation and elevate Paco2.
Note that the relationship between Paco2 and minute volume is nearly
linear. Also note that very high arterial Paco2 tensions depress the
ventilatory response (CO2 nar-cosis). The Paco2 at which
ventilation is zero (x-inter-cept) is
known as the apneic threshold. Spontaneous respirations are typically absent
under anesthesia when Paco2 falls below the apneic threshold. (In
the awake state, cortical influences prevent apnea, so apneic thresholds are
not ordinarily seen.) In contrast to peripheral chemoreceptors , central
chemoreceptor activity is depressed by hypoxia.
3. Peripheral Sensors
Peripheral Chemoreceptors
Peripheral chemoreceptors include the
carotid bod-ies (at the bifurcation of the common carotid arter-ies) and the
aortic bodies (surrounding the aortic arch). The carotid bodies are the
principal periph-eral chemoreceptors in humans and are sensitive to changes in
Pao2, Paco2,
pH, and arterial perfu-sion pressure. They interact with central respiratory
centers via the glossopharyngeal nerves, producing reflex increases in alveolar
ventilation in response to reductions in Pao2,
arterial perfusion, or eleva-tions in [H +] and Paco2.
Peripheral chemoreceptors
are also stimulated by cyanide,
doxapram, and large doses of nicotine. In contrast to central chemore-ceptors,
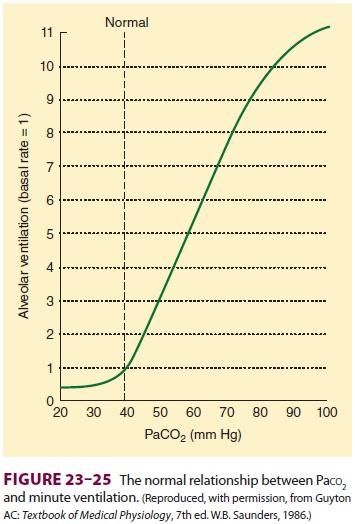
which respond primarily to Paco2 (really [H+]), the carotid bodies are most sensitive to Pao2 (Figure23–26). Note that
receptor activity does not appreciably increase until Pao 2 decreases below 50 mm Hg. Cells of the carotid body
(glomus cells) are thought to be primarily dopaminergic neurons. Anti-dopaminergic
drugs (such as phenothiazines), most commonly used anesthetics, and bilateral
carotid surgery abolish the peripheral ventilatory response to hypoxemia.
Lung Receptors
Impulses from these receptors are
carried centrally by the vagus nerve. Stretch receptors are distributed in the
smooth muscle of airways; they are respon-sible for inhibition of inspiration
when the lung is inflated to excessive volumes (Hering–Breuer
infla-tionnormally play a minor role in humans. In fact, bilat-eral vagal nerve
blocks have a minimal effect on the normal respiratory pattern.
Irritant receptors in the
tracheobronchial mucosa react to noxious gases, smoke, dust, and cold gases;
activation produces reflex increases in respiratory rate, bronchoconstriction,
and coughing. J (juxta-capillary) receptors are located in the inter-stitial
space within alveolar walls; these receptors induce dyspnea in response to
expansion of inter-stitial space volume and various chemical mediators
following tissue damage.
Other Receptors
Th ese include various muscle and joint
receptors on pulmonary muscles and the chest wall. Input from these sources is
probably important during exer-cise and in pathological conditions associated
with decreased lung or chest compliance.
4. Effects of Anesthesia on the Control of Breathing
The most important effect of most
general anesthet-ics on breathing is a tendency to promote hypoven-tilation.
The mechanism is probably dual: central depression of the chemoreceptor and
depression of external intercostal muscle activity. The magnitude of the
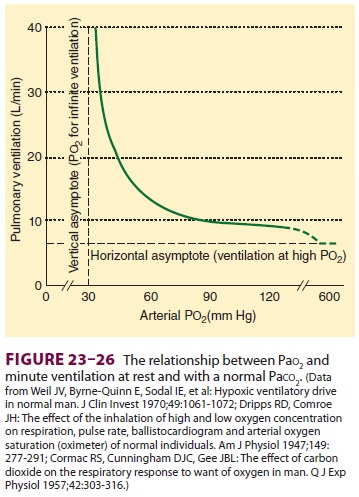
hypoventilation is generally proportional to anesthetic depth. With increasing
depth of anesthesia, the slope of the Paco2/minute ventilation curve
decreases, and the apneic threshold increases (Figure23–27). This effect
is at least par-tially reversed by surgical stimulation.
The peripheral response to hypoxemia is
even more sensitive to anesthetics than the central CO 2 response and is nearly abolished by even
subanes-thetic doses of most inhalation agents (including nitrous oxide) and
many intravenous agents.
Related Topics