Chapter: Medical Immunology: Immunoglobulin Structure
Immunoglobulin Regions and Domains
IMMUNOGLOBULIN REGIONS AND DOMAINS
A. Variable and Constant Regions of the Immunoglobulin Molecule
The light chains of human immunoglobulins are composed of 211–217 amino acids. As mentioned above, there are two major antigenic types of light chains (k and λ). When the amino acid sequences of light chains of the same type were compared, it became evident that two regions could be distinguished in the light-chain molecules: a variable region, comprising the portion between the amino-terminal end of the chain and residues of 107–115, and a constant region, extending from the end of the variable region to the car-boxyl terminus (Fig. 5.7).
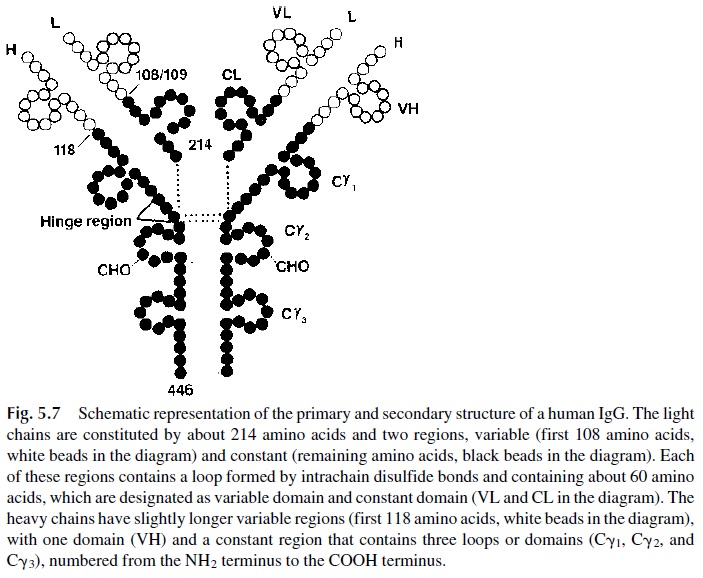
The light-chain constant regions were found to be almost identical in light chains of the same type, but to differ markedly in k and λ chains. It is assumed that the difference in antigenicity between the two types of light chains is directly correlated with the structural differences in constant regions.
In contrast, the amino acid sequence of the light-chain variable regions is different even in proteins of the same antigenic type, and early workers thought that this sequence would be totally individual for any single protein. With increasing data, it became evident that some proteins shared similarities in their variable regions, and it has been possible to classify variable regions into three groups: Vk , Vλ , and VH. Each group has been further subdivided into several subgroups. The light-chain V region subgroups (Vk , Vλ ) are “type” specific, i.e., Vk subgroups are only found in k proteins and Vλ subgroups are always as-sociated with λ chains. In contrast, the heavy-chain V region subgroups (VH) are not “class” specific. Thus, any given VH subgroup can be found in association with the heavy chains of any of the known immunoglobulin classes and subclasses.
The heavy chain of IgG is about twice as large as a light chain; it comprises approx-imately 450 amino acids, and a variable and a constant region can also be identified. The variable region is constituted by the first 113–121 amino acids (counted from the amino
The constant region is almost three times larger; for most of the heavy chains, it starts at residue 116 and ends at the carboxyl terminus (Fig. 5.7). The maximal degree of homology is found between con-stant regions of IgG proteins of the same subclass.
B. Immunoglobulin Domains
The immunoglobulin molecule contains several disulfide bonds formed between contigu-ous residues. Some of them join two different polypeptide chains (interchain disulfide bonds), keeping the molecule together. Others (intrachain bonds) join different areas of the same polypeptide chain, leading to the formation of “loops.” These loops and adjacent amino acids constitute the immunoglobulin domains, which are folded in a characteristic -pleated-sheet structure (Fig. 5.8).
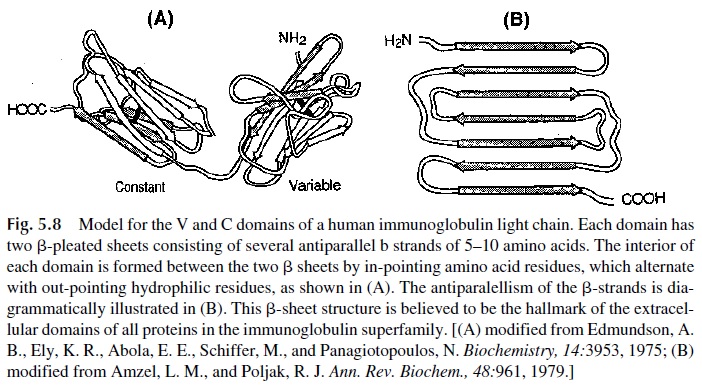
The variable regions of both heavy and light chains have a single domain, which is involved in antigen binding. Light chains have one single constant region domain (CL), while heavy chains have several constant region domains (three in the case of IgG, IgA, and IgD; four in the case of IgM and IgE). The constant region domains are generically desig-nated as CH1, CH 2, and CH 3, or if one wishes to be more specific, they can be identified as to the class of immunoglobulins to which they belong by adding the symbol for each heavy chain class ( γ, α, µ, δ, ε ). For example, the constant region domains of the IgG molecule can be designated as Cγ1, Cγ 2, and Cγ3. Different functions have been assigned to the differ-ent domains and regions of the heavy chains. For instance, Cγ2 is the domain involved in complement fixation, while both Cγ2 and Cγ3 are believed to be involved in the binding to phagocytic cell membranes.
The “hinge region” is located between CH1 and CH2, and its name is derived from the fact that studies by a variety of techniques, including fluorescence polarization, spin la-beling, electron microscopy, and x-ray crystallography, have shown that the Fab fragments can rotate and waggle, coming together or moving apart. As a consequence IgG molecules can change their shape from an “Y” to a “T” and vice versa using the region intercalated between Cγ1 and Cγ2 as a hinge. The length and primary sequence of the hinge regions play an important role in determining the segmental flexibility of IgG molecules. For ex-ample, IgG3 has a 12-amino-acid hinge amino-terminal segment and has the highest seg-mental flexibility. The hinge region is also the most frequent point of attack by proteolytic enzymes. In general, the resistance to proteolysis of the different IgG subclasses is in-versely related to the length of the hinge amino-terminal segments—IgG3 proteins are the most easily digestible, while IgG2 proteins, with the shortest hinge region, are the most re-sistant to proteolytic enzymes.
Related Topics