Chapter: Biotechnology Applying the Genetic Revolution: Transgenic Plants and Plant Biotechnology
Getting Genes into Plants Using the Ti Plasmid
GETTING
GENES INTO PLANTS USING THE TI PLASMID
Plants suffer from tumors,
though these are quite different from the cancers of animals. The most common
cause is the Ti plasmid (tumor-inducing plasmid), which is carried by soil
bacteria of the Agrobacterium group.
Specifically, the Ti plasmid of Agrobacterium
tumefaciens is an important tool for
plant genetic engineering. The most important aspect of the infection is that a specific segment of the Ti plasmid DNA
is transferred from the bacteria to the plant. Scientists have exploited this
genetic transfer in order to get genes with desired properties into plant
cells. Agrobacterium is unique in the
ability to transfer a segment of its DNA from one kingdom to another. Most DNA
transfers occur only between closely related organisms.
In nature, Agrobacterium is attracted to plants
that have minor wounds by phenolic compounds such as acetosyringone, which are
released at the wound (Fig. 14.4). These chemicals induce the bacteria to move
and attach to the plant via a variety of cell surface receptors. The same
inducers activate expression of the virulence genes on the Ti plasmid that are
responsible for DNA transfer to the plant. This is under control of a
two-component regulatory system. At the cell surface, the sensor, VirA, is
autophosphorylated when it detects the plant phenolic compounds. Next, VirA
transfers the phosphate to the DNA-binding protein, VirG, which activates
transcription of the vir genes of the
Ti plasmid. Two of the gene products (VirD1 and VirD2) clip the T-DNA borders to form a single-stranded
immature T-complex. VirD2 then attaches to the 5¢ end of the T-DNA, and bacterial helicases
unwind the T-DNA from the plasmid. The single-stranded gap on the plasmid is
repaired, and the T-DNA is coated with VirE2 protein to give a hollow
cylindrical filament with a coiled structure. This is the mature form of T-DNA
and traverses into the plant.
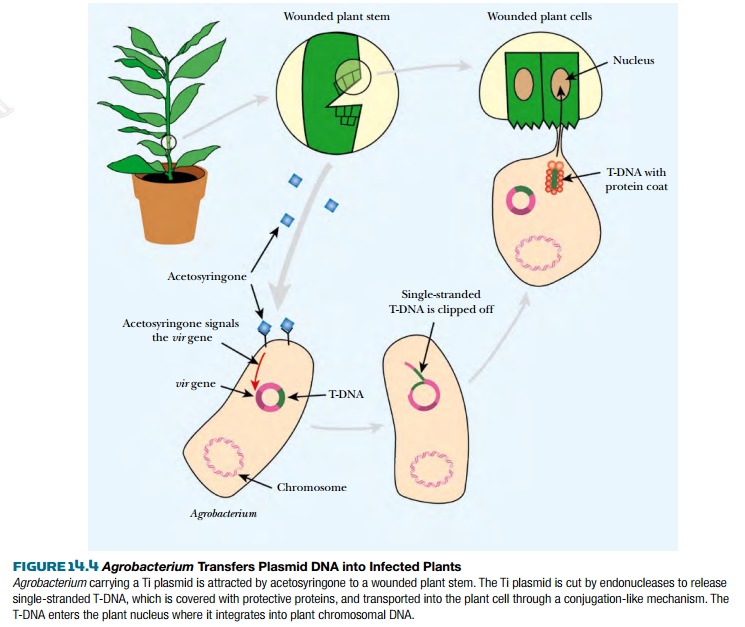
T-DNA is transferred to the
plant in a process similar to bacterial conjugation. First, Agrobacterium forms a pilus. This
rodlike structure forms a connection with the plant cell and opens a channel through which the T-DNA
is actively transported into the plant cytoplasm. Both pilus and transport
complex consist of proteins that are vir
gene products. Once inside the plant cytoplasm, T-DNA is imported into the
nucleus. Both VirE2 and VirD2 have nuclear localization signals that are
recognized by plant cytosolic proteins. These proteins take the T-complex to
the nucleus where it is actively transported through a nuclear pore. The single
T-DNA strand is integrated directly into the plant genome and converted to a
double-stranded form. The integration requires DNA ligase, polymerase, and
chromatin remodeling proteins, which are all supplied by the plant.
Once they are part of the
plant genome, the genes in the T-DNA are expressed. These genes have
eukaryote-like promoters, transcriptional enhancers, and poly(A) sites and
hence are expressed in the plant nucleus rather than in the original bacterium.
The proteins they encode synthesize two plant hormones, auxin and cytokinin.
Auxin makes plant cells grow bigger and cytokinin makes them divide. The
infected plant cells begin to grow rapidly and without control, resulting in a
tumor.
T-DNA also carries genes for
synthesis of opines, which are a variety of different amino acid and sugar
phosphate derivatives. The type of opine differentiates the various strains of Agrobacterium. Opines are made by plant
cells that contain T-DNA but are used by the bacteria as carbon, nitrogen, and
energy sources. Notice how the bacterium tricks the plant
into using its resources to
supply the bacteria with food. The Ti plasmid, which is still inside the Agrobacterium, carries genes that allow
the bacteria to take up these opines and break them down for food. Note that
other bacteria, which might be present by chance, cannot use opines because
they do not possess the genes for uptake and metabolism. This ensures that the
plant feeds only the bacteria with the Ti plasmid.
So how are Ti plasmids used
to improve plants? First, the Ti plasmid is disarmed by cutting out the genes
in the T-DNA for plant hormone and opine synthesis. Then, the transgene of
interest, such as an insect toxin gene, is inserted into the T-DNA region of
the Ti plasmid.
The Ti plasmid is also streamlined
by removing genes that are not involved in moving the T-DNA. These smaller
plasmids are much easier to work with and can be manipulated in Escherichia coli rather than their
original host, Agrobacterium. Now,
when the T-DNA enters the plant cell
and integrates into the chromosome, it will bring in the transgene instead of
causing a tumor.
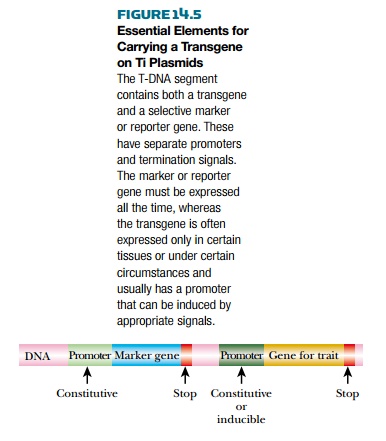
The transferred region of the
plasmid must also have other elements in order for the transgene to function
properly (Fig. 14.5). Expression of the transgene requires a promoter that
works efficiently in plant cells. This may be one of two types. A constitutive promoter will turn the
gene on in all the plant cells throughout development; thus every tissue, even
the fruit or seed, will express the gene. A more refined approach is to use an inducible promoter that has an on/off switch. An example of this is the cab promoter from the gene encoding
chlorophyll a/b binding protein. This promoter is turned on only when the plant
is exposed to light; therefore, root tissues and tubers such as potatoes will
not express the gene. Many different promoters may be used, but ideally, the
promoter should turn on only in tissues that need transgene function. Another
important component for the genetically modified T-DNA region is some sort of
selectable marker. Including an herbicide or antibiotic resistance gene in the
T-DNA region can be used to track whether the foreign DNA has been inserted
into plant cells. The selectable marker may cause problems because it must be
expressed constitutively throughout the plant. Many people worry that the
protein product of the selectable marker could cause allergies or reactions if
expressed in fruit, grain, or vegetables. However, systems exists that can
remove this gene once the transgenic plant has been isolated (see later
discussion).
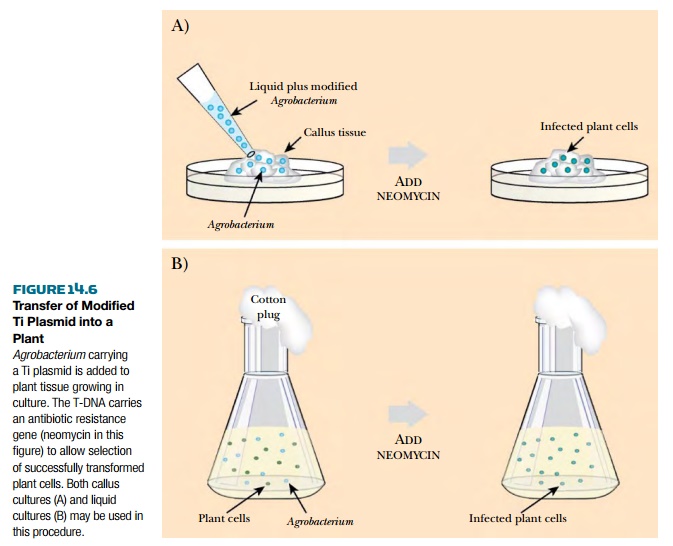
In practice, Agrobacterium is used to transfer genes
of interest into plants using tissue culture. Either dissociated plant cells
called protoplasts or a piece of
callus are cultured with Agrobacterium harboring
a Ti plasmid with modified T-DNA. After coculture, the plant cells are harvested and incubated with
the herbicide or antibiotic used as the selectable marker. This kills all the
cells that were not transformed with T-DNA or failed to express the genes on
the T-DNA. The transformed cells can then be induced to produce shoot and root
tissue by altering the hormone conditions in the medium as described earlier
(Fig. 14.6). The small transgenic plants can then be screened for transgene
expression levels (see later discussion).
Recently, a method for in
planta Agrobacterium transformation
was developed and has revolutionized the plant transformation world. In planta transformation is also known
as the floral dip method. The method
was developed using the model plant Arabidopsis
but has been extended to other plants, such as wheat and maize. First, Arabidopsis plants are grown until
flower buds begin to form. These buds are removed and allowed to regenerate for
a few days. Once they begin to regenerate, the plants are dipped into a
suspension of Agrobacterium containing
a surfactant. The surfactant allows the
Agrobacterium to adhere to the plant and transfer its T-DNA. Because the
flower buds are just beginning to form, the T-DNA somehow becomes part of the
germline through the ovarian tissue. The plant is allowed to finish growing and
set seed. These seeds are harvested and grown in selective media to find those
that have integrated and expressed T-DNA. Although the method gives a low
percentage of transformants, so many seeds can be screened that the overall
procedure works well.
Related Topics