Chapter: Basic Concept of Biotechnology : Biomolecules
Fatty Acids
Fatty Acids
Fatty acids consist of a long carbon chain (also called an acyl chain) with carboxylic acid at one end. The vast majority of fatty acids are unbranched linear molecules. The carboxylic acid is ionized at physiological pH (the carboxyl group is deprotonated and therefore negatively charged). Fatty acids in most biological systems are synthesized by serial addition of two carbon units. As a result, fatty acids usually contain an even number of carbons, especially in animals, which synthesize even-chain fatty acids almost exclusively. Biological fatty acids usually contain 14 to 20 carbons, although small amounts of 22 and 24 carbon compounds are found in some tissues. The fatty acid acyl chain “prefers” to be extended, because this results in the least steric hindrance; however, the chain is very flexible, and will adopt a large variety of conformations. The reason for this flexibility is that each carbon-carbon bond can (more or less) freely rotate, and all fatty acids have many carbon-carbon bonds.
Saturated fat and unsaturated fat
Many fatty acids contain double bonds. Fatty acids that do not contain carbon-carbon double bonds are considered to be saturated. When producing unsaturated fatty acids, the biosynthetic machinery incorporates nearly exclusively cis configuration double bonds. Some food products, such as margarine, may contain trans double bonds because of manipulations during food processing; some evidence suggests that this is a potential health problem, and that food products lacking trans fatty acids are therefore healthier. Monounsaturated fatty acids contain a single double bond, while polyunsaturated fatty acids contain more than one site of unsaturation. The cis bond causes a kink in the fatty acid acyl chain (recall that, unlike single bonds, double bonds do not allow free rotation).
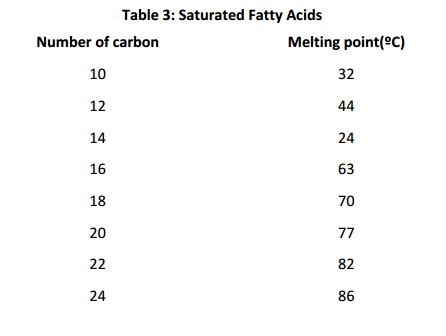
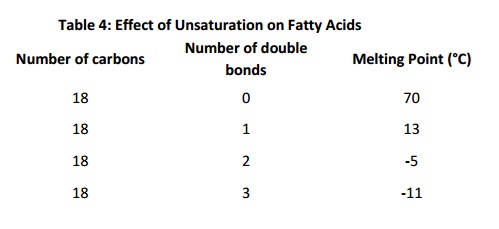
“Partially hydrogenated vegetable oil” is a term often found onfood ingredient labels. It means that some of the fatty acids in the oil were “hydrogenated” (reduced, so that the double bonds were converted to single bonds). Why is this done? Saturated fat and monounsaturated fat forms a solid at room temperature Polyunsaturated fat is (usually) liquid at room temperature. Mixing saturated/monounsaturated/polyunsaturated fatty acids is a way of regulating consistency of food, and also of regulating the consistency of biological systems. This is because, in membranes or in bulk lipid, cis double bonds alter packing density. This decreases van der Waals contacts: the presence of cist double bonds therefore results in lower melting temperature because they result in less regular and less stable structures. The table below shows the effect of chain length and number of cist double bonds on melting temperature (note that the values for melting temperature vary somewhat depending on the reference consulted). Longer chains result in higher melting temperatures;increasing numbers of double bonds decreases melting temperature for fatty acids of a given length
Nomenclature
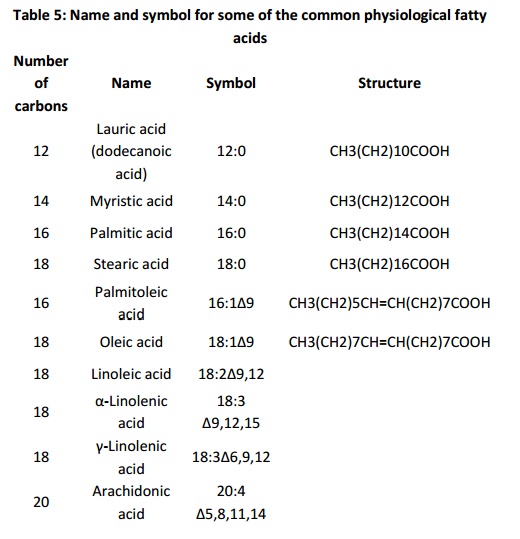
Fatty acids are named using both historical labels and symbols. Both types of nomenclature uniquely define molecules with specific lengths and number and position of double bonds. The table 5 (below)gives the name and symbol for some of the common physiological fatty acids
Simple Lipids The second types of simple lipids are waxes:
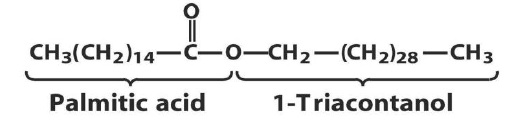
They are the esters of fatty acids with long chain monohydroxy alcohols 26 to 34 carbons atoms. Waxes are wide-spread in nature and occur usually as mixtures. They form a protective coating on the surfaces of animals and plants. Some insects also secrete waxes. The main constituent of bees wax obtained from the honey comb of bees is myricyl palmitate:
Related Topics