Chapter: Basic Radiology : Liver, Biliary Tract, and Pancreas
Exercise: Pancreatic Neoplasm
EXERCISE 11-6.
PANCREATIC NEOPLASM
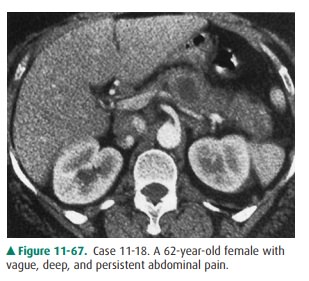
11-18. What is the most likely diagnosis in Case 11-18 (Figure
11-67)?
A.
Pancreatic cyst
B.
Ductal pancreatic carcinoma
C.
Pancreatic metastasis
D.
Peripancreatic lymphadenopathy
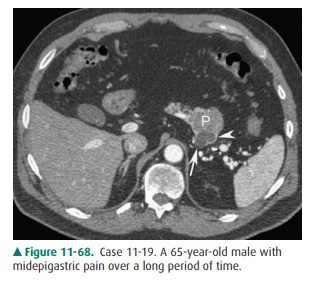
11-19. What is the most likely diagnosis in Case 11-19 (Figure
11-68)?
A.
Cholangiocarcinorna
B.
Cystic pancreatic neoplasm
C.
Ductal pancreatic carcinoma
D.
Pancreatic cyst
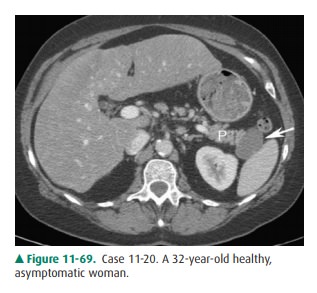
11-20. What is the most likely diagnosis in Case 11-20 (Figure
11-69)?
A.
Acute edematous pancreatitis
B.
Pancreatic pseudocyst
C.
Pancreatic cyst
D.
Cystic pancreatic neoplasm
Radiographic Findings
11-18. In this case, there is a low, but
not fluid, density lesion in the pancreatic body, expanding
the contour of thepancreas, and not associated with any inflammatory changes in
the peripancreatic fat, findings most con-sistent with a ductal adenocarcinoma
(B is the correct answer to Question 11-18).
11-19. In this case, CT demonstrates a fluid density lesion
(arrow) in the tail of the pancreas (P). There is en-hancement along the rim (arrowhead)
that is not as-sociated with surrounding inflammatory change in the
peripancreatic fat. Findings are compatible with a cystic neoplasm of the
pancreas (B is the correct an-swer to Question 11-19).
11-20. In this case, CT demonstrates a unilocular fluid
at-tenuation mass (arrow) in the tail of the pancreas (P) without enhancement
or nodularity. This is difficult to distinguish from a cystic neoplasm;
however, at surgery this was a lymphoepithelial cyst (C is the cor-rect answer
to Question 11-20).
Discussion
Pancreatic masses include tumors,
tumor-like masses such as cysts and developmental anomalies, and inflammatory
le-sions. These can overlap in appearance, as when an inflam-matory mass
simulates a neoplastic mass on imaging studies. They can be causally related,
as when a neoplastic mass sec-ondarily causes an inflammatory mass. Therefore,
differenti-ation among them is not entirely possible, either clinically or
radiographically. However, the prognostic and management implications of the lesions
that create pancreatic masses differ considerably and therefore require
extensive and often invasive investigation. Although contrast studies of the
gastrointesti-nal tract can be used to infer the presence of a mass, usually
cross-sectional imaging studies are employed to establish the diagnosis.
Tumors of the pancreas are
important clinical entities; some have an extremely poor prognosis and some
produce serious clinical symptoms. They can be classified according to origin
as epithelial tumors, endocrine tumors, and mis-cellaneous lesions. Epithelial
tumors can be solid or cystic. Solid ductal adenocarcinoma is the most common
overall and carries the worst prognosis (mean survival 4 months). Cystic tumors
can be divided into cystic lesions arising from the pancreatic parenchymal
cells, such as cystade-noma or cystadenocarcinoma, and those arising from the
pancreatic ductal cells, such as intraductal papillary muci-nous tumors.
Compared to adenocarcinoma, these tumors have a less serious prognosis. Endocrine,
or islet cell, tu-mors elaborate hormonal substances and can create clini-cally
significant symptoms. The two most common of these are insulinoma, which
releases insulin and produces hypoglycemia, and gastrinoma, which releases
gastrin and produces Zollinger-Ellison syndrome. There are many other important
kinds of hormonally active pancreatic en-docrine tumors, and each is designated
by the hormone it secretes (eg, glucagonoma, somatostatinoma). Miscellaneous
lesions arise from pancreatic parenchymal tissue (eg, metas-tases, especially
from melanoma, and lung or breast cancer) or from tissue other than pancreas
(eg, intrapancreatic cholangiocarcinoma or peripancreatic lymph node). These
miscellaneous lesions are important because they sometimes strongly simulate
true pancreatic neoplasms on imaging studies.
Ductal adenocarcinoma has a
variety of appearances on imaging studies. On US, it usually is seen as a
focal, hypoe-choic, irregular, solid mass. Rarely, it is isoechoic or involves
the entire gland. In some pancreatic head masses, the only finding may be that
the uncinate process is rounded. The pancreatic or biliary duct may be dilated
by the obstructing tumor. Pseudocysts, cystic collections in or around the
pan-creas, may form because of pancreatic duct dilatation and per-foration. On
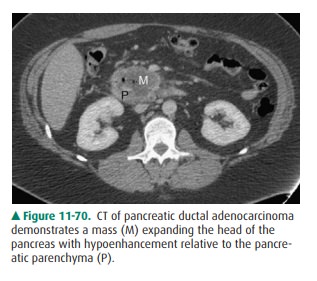
CT, the tumor presents as a solid, low-density, irregular mass, perhaps with
ductal dilatation, pseudocyst for-mation, or both (Figure 11-67). It usually
enhances to a lesser extent than the surrounding pancreas (Figure 11-70).
Occa-sionally, the tumors will enhance brightly. The pancreas distal to a
ductal adenocarcinoma is often atrophic. Angiography may be used to demonstrate
the vascular anatomy and estab-lish definitively whether certain key vessels
(eg, the superior mesenteric artery or vein) are encased. If so, the lesion is
un-resectable. NM currently has no established role in evalua-tion of
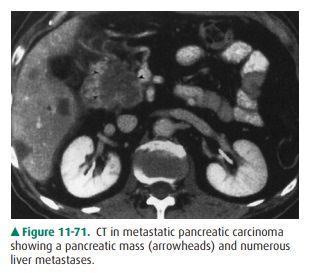
pancreatic tumors. Associated metastases in the liver establish the fact that a
pancreatic mass cannot be simply in-flammatory (Figure 11-71). General
pertinent negatives on cross-sectional imaging may help to differentiate
adenocarci-noma from other nontumorous masses. Calcification is rarely, if
ever, seen in ductal adenocarcinoma, and it is almost never hypervascular.
Ductal adenocarcinoma is
simulated by a number of other entities. These include peripancreatic
lymphadenopa-thy, intrapancreatic cholangiocarcinoma, and pancreatic
metastases.
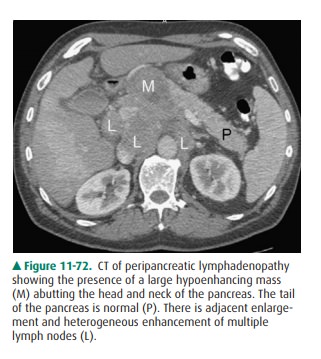
Peripancreatic lymphadenopathy
from lymphoma, leukemia, or any other primary malignancy can closely resem-ble
a pancreatic mass. On imaging studies it may appear as solid soft tissue in the
pancreatic region (Figure 11-72). Keys todifferentiating lymphadenopathy from a
primary solid mass include smooth lobulation and pseudoseptations caused by
in-complete coalescence of the lymph nodes. Also, peripancreatic
lymphadenopathy is much less likely to obstruct the pancreatic duct, although
suprapancreatic lymph nodes obstruct the bil-iary duct as it passes through the
porta hepatis.
Two uncommon neoplastic processes
that occur in the pancreas are cholangiocarcinoma and metastases.
Cholangio-carcinoma usually does not occur within the pancreas, but when it
does, it can exactly mimic a pancreatic head mass to the extent of producing
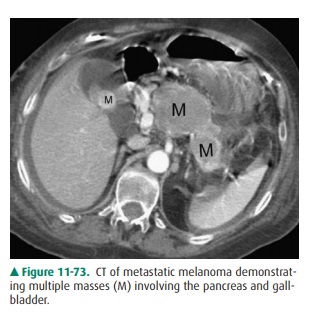
both the pancreatic and common bile duct dilatation. Metastases appear as solid
intrapancreatic le-sions, but with necrosis, they appear as fluid masses.
Because they may be completely indistinguishable from primary tu-mors, the
diagnosis may be inferred only from the clinical his-tory. Pancreatic
metastases are quite uncommon, usually arise from melanoma or lung primary
lesions, and mimic a focal mass lesion of any neoplastic origin (Figure 11-73).
Pancreatic endocrine tumors may
also simulate ductal adenocarcinoma, and in fact, no specific features
consistently distinguish the two. Occasionally, however, certain imaging
features can be helpful, especially when combined with the history. Many islet
cell tumors appear simply as solid masses within the pancreas. However, some
(especially in insuli-noma) may appear hypervascular when studied with fast
bolus or dynamic CT, and they may appear as extremely dense lesions immediately
after enhancement with intravenous con-trast material. Calcifications, which
sometimes are very dense, are more commonly seen with islet-cell tumors. MR
imaging may have a role in the evaluation of islet-cell tumors, because these
tumors have a characteristic appearance on MR studies
Islet-cell tumors and their
metastases have extremely high signal intensity on T2-weighted MR imaging,
which can be used to characterize the origin of the lesion.
Primary cystic pancreatic
malignancies and pancreatic cysts are not readily confused with typical ductal
adenocarci-noma. Currently, cystic pancreatic masses are classified accord-ing
to whether they arise from parenchymal cells or ductal cells. Cystic
malignancies arising from pancreatic parenchymaare classified as either serous
or mucinous cystic neoplasms. This classification is helpful, because the two
lesions are distin-guishable from each other and from solid lesions on imaging
studies. Serous cystadenomas are usually composed of innu-merable very small
cysts (1 mm to 2 cm). Sometimes they con-tain highly vascularized fibrous septa
and a central stellate fibrotic scar, which may calcify. They are generally
benign. Mu-cinous cystic neoplasms are composed of unilocular or multi-locular
cysts larger than 5 cm and may have large papillary excrescences. They are
considered malignant or premalignant lesions. Both serous cystadenomas and
mucinous cystic neo-plasms are cystic, but differences in the typical sizes of
the cysts can be recognized on US or CT. Cystic malignancies arising from
pancreatic ductal epithelial cells are called intraductal papillary mucinous
tumors. These tumors contain consider-able mucus and therefore exhibit complex
appearances on MR imaging. MRCP can be helpful to demonstrate communica-tion
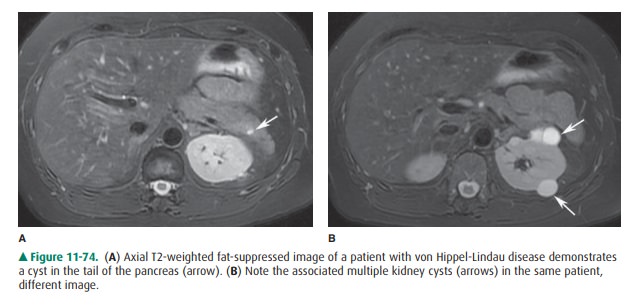
with the pancreatic duct. Pancreatic cysts can occur as iso-lated congenital
cysts or as part of a more generalized multiorgan process that includes adult
polycystic disease or von Hippel-Lindau disease (Figure 11-74). Regardless,
their appearance is similar to that of a cyst in any other organ (Figure
11-69). US and CT depict a uniloculated or multiloc ulated cyst. A pancreatic
cyst can be very difficult to differenti-ate from a mucinous cystic neoplasm.
Management is contro-versial, and they are often followed even when small.
Related Topics