Chapter: Modern Medical Toxicology: Neurotoxic Poisons: Inebriants
Ethylene Glycol - Inebriant Neurotoxic Poisons
Ethylene Glycol
Synonyms
·
1,2-Ethanediol; Glycol alcohol.
Physical Appearance
·
Colourless, syrupy, odourless,
non-volatile liquid, with a bitter-sweet taste.
Uses
· Antifreeze: Ethylene glycol lowers the freezing point ofwater. More than 25% of the ethylene glycol produced is used in antifreeze and coolant mixtures for motor vehicles.
· It is also used widely for aircraft deicing, and used in condensers and heat exchangers.
· Solvent.
· Hydraulic brake fluid.
· As a glycerine substitute in
commercial products such as paints, lacquers, detergents, and cosmetics.
Usual Fatal Dose
·
About 70 to 100 ml (1.4 ml/kg or
1.56 gm/kg).
Mode of Action
Ethylene
glycol is not absorbed through skin, and because of its low vapour pressure
does not produce toxicity upon inhala-tion. It is however rapidly absorbed
through the GI tract and is metabolised (more than 80%) to glycoaldehyde,
glycolic acid, and oxalic acid which inhibit diverse metabolic pathways in the
body, including oxidative phosphorylation. Other metabolites include glyoxylic
acid, glyoxal, formic acid, glycine, oxaloma-late, malate, benzoic acid, and
hippuric acid.
Clinical Features
First Phase (CNS
stage): upto 12 hours post-ingestion.
·
This stage is mainly due to the parent compound itself and
is characterised by vomiting, inebriation, lethargy, nystagmus, ataxia,
convulsions, and coma.
·
Facial paralysis, strabismus, ophthalmoplegias, papil-loedema,
mydriasis, retinal injury, and eye and throat irritation may occur.
Second Phase (CVS
stage): 12 to 24 hours post-ingestion.
·
This stage is characterised by tachycardia hypertension
(sometimes hypotension), tachypnoea, congestive heart failure, and circulatory
collapse.
·
Severe metabolic acidosis with compensatory
hyper-ventilation can develop with multiple organ failure in significant
poisonings. Tachypnoea and Kussmaul’s respiration may be the first clinical
signs of developing metabolic acidosis which, if untreated, can progress and
become life-threatening.
·
Cardiogenic pulmonary oedema may occur with severe
poisoning.
Third Phase (Renal
stage): 24 to 72 hours post-ingestion.
·
There is oliguria, flank pain, acute tubular necrosis and
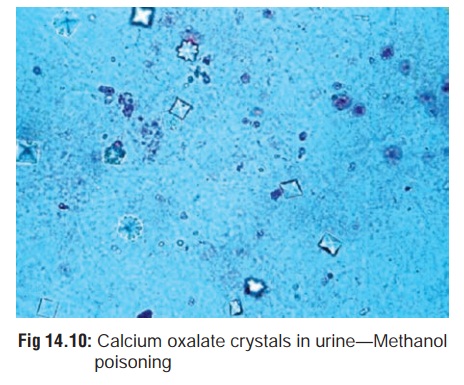
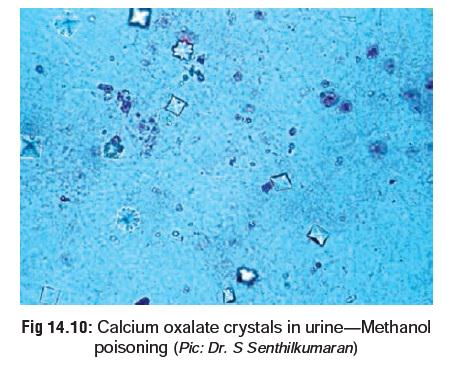
renal failure. Urine contains calcium oxalate or hippurate crystals. Calcium
oxalate crystals are found as monohydrates (prism or needle-like) or dihydrates
(tent or envelope-shaped). The former may resemble sodium urate crystals.
Hippurate crystals are produced by the transamination of glyoxalate to glycine.
It is important to note that absence of calcium oxalate crystals does not rule
out the diagnosis. Haematuria and proteinuria are common. In surviving cases,
renal function usually returns to normal, but in some cases permanent renal
damage has occurred.
·
Hypocalcaemia results in manifestations of tetany.
·
Delayed onset of adult respiratory distress syndrome (ARDS)
has been described after ingestion of ethylene glycol.
Diagnosis
· High anion gap acidosis: Increased
anion gap metabolic acidosis results from the metabolism of ethylene glycol to
acidic metabolites, predominantly glycolic acid.
· Osmolal gap: Normal anion gap is 12
to 16 using the formula AG = (Na + K) – (Cl + HCO3), but may vary from
laboratory to laboratory.The osmolal gap may be used to estimate the serum
ethylene glycol level (in mg/100 ml) by simply multiplying the gap by 6.2 (the
molecular weight of ethylene glycol/10). This method assumes that the patient’s
serum contains only ethylene glycol (and no other osmoti-cally active
substances such as ethanol).
· Calcium oxalate crystals in urine (Fig 14.10).
· Xanthochromic CSF with pleocytosis.
· Determine blood ethylene glycol
concentration in all patients. Ethylene glycol concentrations must be
inter-preted with regard to the time of ingestion and the acid/ base status of
the patient. Shortly after ingestion ethylene glycol concentrations greater
than 30 to 50 mg/100 ml (8.06 mmol/L) are frequently associated with severe
intoxication. In severely acidotic or acidaemic patients lower ethylene glycol
concentrations may be associated with severe toxicity.
· If antifreeze has been ingested, the
urine will fluoresce from the fluorescent dye in the product, when examined
under Wood’s lamp. A fluorescent dye, sodium fluorescein, is present in many
commercial antifreeze products. However, fluorescent urine is not a reliable
indicator of ethylene glycol ingestion, due to variations in interpretation of
urine fluorescence among observers and the fact that most normal urine
specimens exhibit some degree of fluorescence.
a.
Method:
–– If the fluorescein content is not
listed on the container, a sample of the product should also be examined for
fluorescence.
–– Urine samples should be collected as soon as possible after ingestion, preferably within 2 hours and absolutely within 4 hours. A spectrofluoropho-tometer is more sensitive than visual inspection.
–– Urine must be collected using
non-fluorescent containers (i.e. borosilicate glass test tubes). Plastic
specimen containers are fluorescent.
–– The pH of the urine should be
checked and adjusted to 4.5 or greater before examination.
Treatment
DO
NOT WAIT FOR SYMPTOMS TO APPEAR.
1.
Stomach wash and activated charcoal.
However, the utility of activated charcoal is limited due to ethylene glycol’s
rapid absorption from the GI tract and its poor binding affinity for activated
charcoal. Unless there is concern for coingestants, there is little benefit
from acti- vated charcoal administration in ethylene glycol inges- tions.
2.
Ethanol is the antidote and must be
given IV, (same as for methanol poisoning). It inhibits the metabolism of
ethylene tive.
a. Indications: The
following criteria have been proposed by the American Academy of Clinical
Toxicology as with an antidote (either ethanol or fomepizole):
–– Documented plasma ethylene glycol
concentration greater than 20 mg/100 ml
Or
–– Documented recent (hrs.) history of ingesting
toxic amounts of ethylene glycol and osmolal gap greater than 10 mOsm/L
Or
––
History or strong clinical suspicion of ethylene glycol poisoning and at least
2 of the following criteria:
-- Arterial pH less than 7.3
--
Serum bicarbonate less than 20 mEq/L -- Osmolal gap greater than 10 mOsm/L --
Urinary oxalate crystals present.
b. Loading Dose (ethanol):
–– Intravenous Loading Dose
--
Administer 7.6 ml/kg IV of 10% ethanol (V/V) in dextrose 5% in water over 30
minutes to achieve a blood ethanol concentration of above 100 mg/100 ml (21.7
to 28.2 mmol/L). Some authors recommend a loading dose of 10 ml/ kg to ensure
an adequate initial level despite variability in ethanol distribution and
ongoing metabolism during the infusion.
–– Oral Loading Dose
--
95% ethanol: Administer 0.8 to 1 ml/kg orally in 6 ounces of orange juice over
30 minutes.
--
40% ethanol: Administer 1.8 to 2 ml/kg orally in 6 ounces of orange juice over
30 minutes (80° proof spirits contain 40% ethanol; for 20% (40° proof) spirits
administer 4 ml/kg).
c. Maintenance Dose (ethanol):
––
Dosing to maintain a blood ethanol level of 100 mg/100 ml (21.7
millimoles/litre). Begin main-tenance infusion as soon as the loading dose is
infused.
––
Determine blood ethanol concentrations at the end of the loading dose and
hourly thereafter until stable levels of 100 to 120 mg/100 ml have been
achieved. Monitor blood ethanol concentrations at least three times daily once
a stable ethanol infusion has been achieved.
––
Patients who have concurrently ingested ethanol and ethylene glycol may have a
normal acid-base profile and urinalysis despite a dangerously elevated blood
ethylene glycol concentration. Consider implementing the ethanol treatment
regimen in these patients until an ethylene glycol concentra-tion can be
determined. Determine blood ethanol concentration before beginning antidotal
therapy and modify the loading dose accordingly.
d. Dose (fomepizole): An
initial loading dose of 15 mg/kgis intravenously infused over 30 minutes
followed by doses of 10 mg/kg every 12 hours for 4 doses, then 15 mg/kg every
12 hours until ethylene glycol concentra-tions are below 20 mg/100 ml.
3.
Haemodialysis*:
Indications –
––
Severe metabolic acidosis (< 7.25-7.3) unrespon-sive to therapy
–– Renal failure
–– Blood ethylene glycol 50 mg/100 ml (8.06
millimoles/L) unless fomepizole is being given and patient is asymptomatic with
normal arterial pH –– Deteriorating
vital signs despite intensive supportive therapy
––
Electrolyte imbalances unresponsive to conven-tional therapy
–– Serum glycolic acid level > 8 mmol/L.
4.
Sodium bicarbonate IV.
5.
Pyridoxine 50 mg and thiamine 100 mg IM, 6th hourly for 2 days. Thiamine is
recommended to stimulate the conver-sion of glyoxylate to
alpha-hydroxy-beta-ketoadipate, a non-toxic metabolite. Pyridoxine is
recommended to allow adequate stores of cofactor necessary for the conversion
of glyoxylate to nontoxic glycine.
6.
Monitor serum calcium level and replace as indicated, with 10% calcium
gluconate IV.
7. Maintenance of good urine volume enhances urinary elimi-nation of ethylene glycol.
Autopsy Features
·
Cerebral oedema, chemical
meningoencephalitis.
·
Toxic damage of liver and kidneys.
·
Oxalate crystals in brain, spinal
cord, and kidneys.
Related Topics