Chapter: Modern Medical Toxicology: Neurotoxic Poisons: Inebriants
Ethanol - Inebriant Neurotoxic Poisons
Ethanol
Synonyms
·
Ethyl alcohol; Grain alcohol.
Physical Appearance
Clear, colourless liquid with a faint fruity odour, and
sweetish burning taste. It is both water soluble and lipid soluble.
Sources
·
Ethanol is produced mostly by
synthetic production from ethylene. This is mainly by direct hydration process
(replacing the earlier method of indirect hydration using sulfuric acid).
·
Fermentation of sugar, cellulose, or
starch: Such is the method used in the production of beverage alcohol.
·
Enzymatic hydrolysis of cellulose.
·
Ethanol can also be obtained by the
reaction of methanol with synthesis gas at 185°C and under pressure.
·
Anhydrous ethanol is manufactured by
azeotropic distil-lation.
·
Beverage ethanol is produced by
fermentation of a sugar (from cereal, vegetable, or fruit) with yeast. If
cereal is Malt is produced by moistening barley and allowing it to sprout which
is then dried, ground, and added to the cereal in water resulting in the
formation of mash. Beer is brewed by
filtering mash and treating the
filtered liquid ( wort) with yeast.
Whisky is made by adding yeast directly to the malted mash. Strong alcoholic beverages are distilled after fermentation.
o The
ethanol content of various alcoholic beverages is expressed by volume percent
or by proof, the latter being twice the percentage of
alcohol by volume. Proofspirit refers to a standard mixture of alcohol and
water of relative density 12/13 at 51°F, i.e. 49.28% of alcohol
by weight (or 57.10% by volume). Proof strength of alcoholic beverages is
expressed in degrees.
o
Table
14.1 lists the ethanol content of various alcoholic beverages.
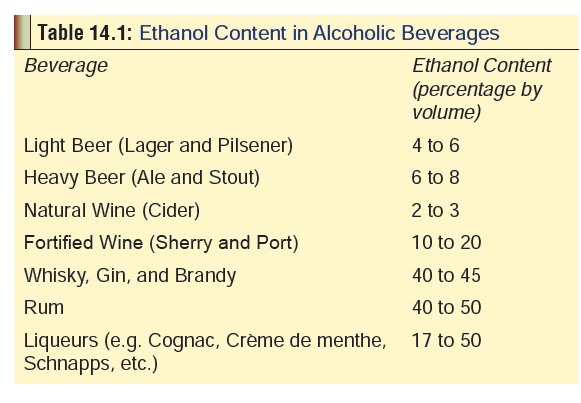
o Apart
from ethanol however, these beverages also contain several congeners to varying
extent, e.g. low molecular weight alcohols such as methanol and butanol,
as well as aldehydes, esters, phenols, tannins,and heavy metals (lead, cobalt,
iron, etc.). Vodka is the purest form and contains no
congeners. It is virtually odourless. White rum is also relatively pure.
Uses
·
Beverage—Popular
alcoholic beverages include beer,wine, whisky, gin, brandy, rum, and vodka (Table 14.1) In
addition there are several indigenous preparations peculiar to particular
regions, e.g. arrack, toddy, and feni in India, tequila in Mexico, sake in
Japan, eau de vie or fruit brandy in
France, etc.
·
Solvent for after-shaves, colognes,
mouthwashes, and perfumes. The alcohol content in these is variable (15 to 80
%).
·
Medicinal—
o Several
antihistaminic, decongestant, multivitamin, and cough syrups contain varying
percentage of alcohol (2 to 25 %).
o Ethanol
has been popular in the past as an antiseptic. Surgical spirit used even today
is mostly ethanol with a small quantity of methanol (90 to 95% and 5 to 10 %
respectively), along with traces of castor oil and methyl salicylate.
o Ethanol
sponging is an effective remedy for hyper-
o Injection
of dehydrated alcohol (absolute alcohol) in close proximity of nerves or
sympathetic ganglia is said to be effective for the relief of long lasting pain
in
o Antidote
for methanol and ethylene glycol.
·
Preservative—Rectified spirit (90 to
95 % ethanol) is used
·
as a preservative for viscera, for
chemical analysis. Fuel.
·
Ethanol is used to extract nucleic
acids from whole tissue or tissue culture in virtually all biotechnology
processes.
Usual Fatal Dose
·
One pint (approximately 550 ml) or quart (two pints or
approximately 1100 ml) of a strong distilled spirit such as whisky taken in a
short span of time can be lethal.
·
The usual fatal dose corresponds to approximately 6 grams of
ethanol/Kg body weight (adult); 3 gm/Kg (child).
·
In terms of blood alcohol, a level in excess of 400 to 500
mg/100 ml is usually considered to be lethal. However there is a great deal of
controversy regarding this since there are case reports of individuals
succumbing to much lower blood alcohol concetration (BAC), while there have
been reports of survival even with a BAC of over 1000 mg%.
Toxicokinetics
·
Ethanol is toxic by oral, inhalation, subcutaneous, intra -
venous, intra-arterial, intraperitoneal, and dermal routes.
·
Following oral administration, ethanol is rapidly absorbed
from the stomach (20%) and small intestine (80%). Maximum or peak alcohol
concentration in blood is reached in 30 to 90 minutes following the last drink.
Many factors can delay absorption: undiluted ethanol (by provoking
pylorospasm), presence of food, delayed gastric emptying due to any cause, and
presence of congeners in alcohol. Vapourised ethanol can be rapidly absorbed by
inhalation leading to intoxication. Following an equivalent dose of ethanol,
women achieve a higher blood alcohol level than do men as a result of decreased
gastric alcohol dehydroge-nase activity. It is also a fact that liver damage
occurs after consumption of relatively smaller quantities of alcohol in women
as compared to men.
·
More than 90% of ethanol ingested is metabolised in the
body, and only 5 to 10% is excreted unchanged by the kidneys, lungs, and sweat.
Excretion of ethanol by the lungs obeys Henry’s
Law :the ratio between the
concentration of ethanol in the alveolar air and the blood is constant.
This alveolar air/blood constant (1 : 2100) forms the basis for reliably
estimating blood alcohol concentration by breath analysis. Metabolism of
alcohol is accomplished through 3 pathways in the liver—
1.
Alcohol dehydrogenase pathway (in
the cell cytosol): This is the main pathway, by which hydrogen is trans- ferred
from ethanol to nicotine adenine dinucleotide (NAD), reducing it to NADH. The
ratio of NAD to NADH (redox potential) is therefore dramatically altered which
contributes to the development of metabolic abnormalities such as alcoholic
ketoaci- dosis, impaired gluconeogenesis, and alterations in lipid metabolism.
The acetaldehyde that is formed isconverted to acetic acid by aldehyde
dehydrogenase, which in turn is converted to acetylcoenzyme A and enters the
Krebs (citric acid) cycle where it is metabolised to carbondioxide and water.
2.
2. Microsomal ethanol oxidising
system (MEOS, located on the endoplasmic reticulum): This system The ability of
ethanol to stimulate the MEOS system forms the basis for interactions between
ethanol and a number of other drugs metabolised by this system. Half-lives of
several drugs are shortened in chronic alcoholics because of accelerated
metabolism, e.g. phenytoin, methadone, tolbutamide, isoniazid, warfarin, etc.
There are also indications that chronic ethanol abuse may potentiate
paracetamol hepatotoxicity.
3.
3. Peroxidase-catalase system (in
the hepatic peroxi-somes).
4.
In adults, the average rate of
ethanol metabolism is 100 to 125 mg/kg/hr in occasional drinkers, and upto 175
mg/kg/hr in habitual drinkers. The blood alcohol level generally falls at a
rate of 15 to 20 mg/100 ml/hr. This may be higher (upto 30 mg/100 ml/hr) in
chronic alcoholics.
Mode of Action
·
Till recently it was postulated that ethanol depresses the
CNS by dissolving in the cell’s lipid membrane and causing disorganisation of
the lipid matrix (membrane fluidisation). However this mechanism has been
challenged by studies which demonstrated that such membrane fluidisation
occurred only at ethanol concentrations much above the pharmacologic range, and
also that the same changes can be produced by minor temperature changes which
produce no signs of intoxication.
·
Now there are two theories which are gaining
popularity.
o
According to one, ethanol acts by enhancing gama aminobutyric acid (GABA)-nergic function
through ![]() interaction with GABA A receptors and associatedchloride ion
channels. However some investigators arenot convinced by this theory.
interaction with GABA A receptors and associatedchloride ion
channels. However some investigators arenot convinced by this theory.
o
The second theory which appears to be more convincing has to
do with N-methyl-d-aspartate (NMDA) ligand-gated, glutamate receptors. NMDA
receptors mediateneurotoxicity by increasing permeability to calciumand
regulate neuronal long-term potentiation. Studiesdemonstrate that in the acute
form of ethanol use,NMDA receptor function is inhibited, while chronic ethanol
use results in up-regulation of NMDA receptors.
· Pharmacological effects of ethanol—
o
CNS: Ethanol is a CNS depressant but produces some
apparently stimulating effects initially because ofdepression of inhibitory
control mechanisms in thebrain. First, those mental processes which depend on
training and previous experience are affected (memory, concentration, and
insight). Later the person becomesexpansive, garrulous and may demonstrate
emotionallability with mood swings. There are accompanying sensory and motor
disturbances. With severe intoxica- tion there is general impairment of CNS
function, and finally coma supervenes. Acute ethanol use at bedtime interferes
with normal sleep pattern, and with chronicuse marked fragmentation of sleep
occurs.
o
CVS: In moderate doses, ethanol produces tachycardia and
vasodilation of cutaneous vessels with resultant warmand flushed skin. However
no beneficial increase in coro- nary blood flow occurs; in fact there may be
appreciable vasoconstriction which can aggravate existing angina. In spite of
this fact, recent studies have indicated that regular use of moderate amounts
of ethanol is associated with reduced risk for coronary heart disease.
o
GIT: Ethanol normally stimulates salivary and gastric
secretions, but if the concentration is too high (> 40%) they are inhibited,
and the GI mucosa becomes congested and inflamed leading to erosive gastritis.
Regular intake of excessive amounts of ethanol leads to chronic gastritis,
pancreatitis, and cirrhosis of liver.
o Genito-urinary: Ethanol induces
diuresis by inhibition of antidiuretic hormone (ADH). There is a
popularmisconception (perpetuated in pulp fiction and films) that ethanol is an
aphrodisiac. While it is true that there is often enhanced sexual inclination
with (sometimes)aggressive behaviour, this is due to loss of inhibition and
restraint rather than the result of sexual stimulation. Objective measurements
in human beings of peniletumescence and vaginal pressure show that
ethanolactually significantly decreases sexual responsiveness in both men and
women. Chronic ethanol consumption can lead to impotence, sterility, testicular
atrophy, and gynaecomastia (because of hyperestrogenisation, and reduced
production as well as enhanced metabolic inactivation of testosterone). In
women, there is increasedpredisposition to breast
cancer
Related Topics