Chapter: Medical Surgical Nursing: Assessment and Management of Patients With Allergic Disorders
Diagnostic Evaluation of Patients With Allergic Disorders
Diagnostic Evaluation
Diagnostic evaluation of the patient with
allergic disorders com-monly includes blood tests, smears of body secretions,
skin tests, and the radioallergosorbent test (RAST). Results of laboratory
blood studies provide supportive data for various diagnostic pos-sibilities;
however, they are not the major criteria for the diagno-sis of allergic
disease.
COMPLETE BLOOD COUNT WITH DIFFERENTIAL
The white blood cell (WBC) count is usually
normal except with infection. Eosinophils, granular leukocytes, normally make
up 1% to 3% of the total number of WBCs. A level between 5% and 15% is
nonspecific but does suggest allergic reaction. Higher levels are considered
moderate and severe. In moderate eosinophilia, 15% to 40% of blood leukocytes
as eosinophils are found in patients with allergic disorders as well as in
patients with malig-nancy, immunodeficiencies, parasitic infections, and
congenital heart disease, and those receiving peritoneal dialysis. In severe
eosinophilia, 50% to 90% of blood leukocytes as eosinophils are found in the
idiopathic hypereosinophilic syndrome.
EOSINOPHIL COUNT
An actual count of eosinophils may be
obtained from blood sam-ples or smears of secretions. A total eosinophil count
can be ob-tained from a blood sample by using special diluting fluids that
hemolyze erythrocytes and stain the eosinophils. During symp-tomatic episodes,
smears obtained from nasal secretions, con-junctival secretions, and sputum of
atopic patients usually reveal eosinophils, indicative of an active allergic
response.
TOTAL SERUM IMMUNOGLOBULIN E LEVELS
High
total serum IgE levels support the diagnosis of atopic dis-ease. A normal IgE
level, however, does not exclude the diagno-sis of an allergic disorder. IgE
levels are not as sensitive as the paper radioimmunosorbent test (PRIST) and
the enzyme-linked immunosorbent assay (ELISA). Indications for determining IgE
levels include the following:
· Evaluation of
immunodeficiency
· Evaluation of drug
reactions
· Initial laboratory
screening for allergic bronchopulmonary aspergillosis
· Evaluation of allergy
among children with bronchiolitis
· Differentiation of
atopic and nonatopic eczema
· Differentiation of
atopic and nonatopic asthma and rhinitis
SKIN TESTS
Skin
testing entails the intradermal injection or superficial appli-cation
(epicutaneous) of solutions at several sites. Depending on the suspected cause
of allergic signs and symptoms, several dif-ferent solutions may be applied at
several separate sites. These so-lutions contain individual antigens
representing an assortment of allergens, including pollen, most likely to be
implicated in the pa-tient’s disease. Positive reactions (wheal and flare) are
clinically significant when correlated with the history, physical findings, and
results of other laboratory tests.
The results of skin tests complement the data obtained from the history. They indicate which of several antigens are most likely to provoke symptoms and provide some clue to the inten-sity of the patient’s sensitization. The dosage of the antigen (al-lergen) injected is also important. Most patients are hypersensitive to more than one pollen. Under testing conditions, they may not react (although they usually do) to the specific pollens that induce their attacks.
In
cases of doubt about the validity of the skin tests, a RAST or a provocative
challenge test may be performed. If a skin test is indicated, there is a
reasonable suspicion that a specific allergen is producing symptoms in an
allergic patient. Several precau-tionary steps, however, must be observed
before skin testing with allergens:
· Testing is not performed
during periods of bronchospasm.
· Epicutaneous tests
(scratch or prick tests) are performed be-fore other testing methods in an
effort to minimize the risk of systemic reaction.
· Emergency equipment must
be readily available to treat anaphylaxis.
Types of Skin Tests
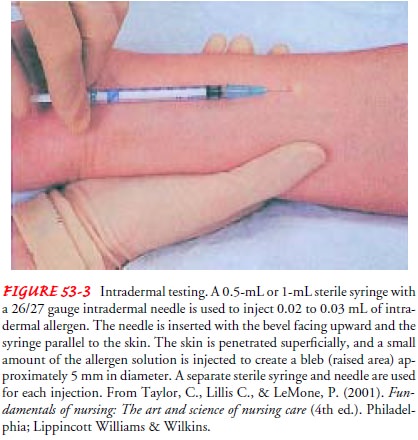
The methods of skin testing include prick
skin tests, scratch tests, and intradermal skin testing (Fig. 53-3). After
prick or scratch tests, intradermal skin testing is performed with allergens
that did not elicit positive reactions. Because a larger antigen challenge is
being used, local or systemic reactions could occur if the same antigens that
produced positive skin or scratch reactions are used. The back is the most
suitable area of the body for skin testing be-cause it permits the performance
of many tests. The multitest ap-plicator is a commercially available device
with multiple test heads that allows simultaneous administration of antigens by
multiple punctures at different sites.
Interpretation of Skin Test Results
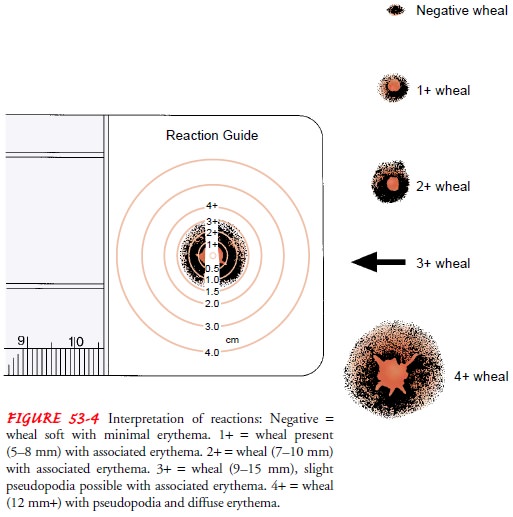
Familiarity with and consistent use of a
grading system are es-sential. The grading system used should be identified on
a skin test sheet for later interpretation. A positive reaction, evidenced by
the appearance of an urticarial wheal (round, reddened skin elevation) (Fig. 53-4),
localized erythema (diffuse redness)
in the area of inoculation or contact, or pseudopodia (irregular projection at
the end of a wheal) with associated erythema is considered indicative of
sensitivity to the corresponding antigen.
There
may be false-negative results due to improper tech-nique, outdated allergen
solutions, and prior use of medications that suppress skin reactivity.
Corticosteroids and antihista-mines, including allergy medications, suppress
skin test reactiv-ity and are usually withheld 48 to 96 hours before testing,
depending on the duration of their activity. False-positive skin tests may
result from improper preparation or administration of allergen solutions.
Interpretation of positive or negative skin tests must be based on the history, physical examination, and other laboratory test results. The following guidelines are used for the interpretation of skin test results:
· Skin tests are more
reliable for diagnosing atopic sensitivity in patients with allergic
rhinoconjunctivitis than in patients with asthma.
· Positive skin tests
correlate highly with food allergy.
· The use of skin tests to diagnose immediate hypersensitivity to
medications is limited because metabolites of medica-tions, not the medications
themselves, are usually responsi-ble for causing hypersensitivity.
PROVOCATIVE TESTING
Provocative testing involves the direct
administration of the sus-pected allergen to the sensitive tissue, such as the
conjunctiva, nasal or bronchial mucosa, or gastrointestinal tract (by ingestion
of the allergen) with observation of target organ response. This type of
testing is helpful in identifying clinically significantaller-gens in patients
with a large number of positive tests.Major dis-advantages of this type of
testing are the limitation of one antigen per session and the risk of producing
severe symptoms, particu-larly bronchospasm, in patients with asthma.
RADIOALLERGOSORBENT TEST
RAST is a radioimmunoassay that measures
allergen-specific IgE. A sample of the patient’s serum is exposed to a variety
of sus-pected allergen particle complexes. If antibodies are present, they will
combine with radiolabeled allergens. After the serum is cen-trifuged,
radioimmunoassay detects the allergen-specific IgE an-tibody. Test results are
then compared with control values. In addition to detecting an allergen, RAST
indicates the quantity of allergen necessary to evoke an allergic reaction.
Values are re-ported on a scale from 0 to 5. Values of 2+ or greater are
consid-ered significant. The major advantages of RAST over other tests include
decreased risk of systemic reaction, stability of antigens, and lack of
dependence on skin reactivity modified by medications. The major disadvantages include the
limited allergen se-lection, reduced sensitivity compared with intradermal skin
tests, lack of immediate results, and cost.
Related Topics