Chapter: Medical Surgical Nursing: Assessment of Neurologic Function
Diagnostic Evaluation of Neurologic Function
Diagnostic Evaluation
COMPUTED TOMOGRAPHY SCANNING
Computed
tomography (CT) makes use of a narrow x-ray beam to scan the head in successive
layers. The images provide cross-sectional views of the brain, with
distinguishing differences in tis-sue densities of the skull, cortex,
subcortical structures, and ventricles. The brightness of each slice of the
brain in the final image is proportional to the degree to which it absorbs
x-rays. The image is displayed on an oscilloscope or TV monitor and is
photographed and stored digitally (Hinkle, 1999a).
Lesions in the brain are seen as variations in
tissue density dif-fering from the surrounding normal brain tissue.
Abnormalities of tissue indicate possible tumor masses, brain infarction,
dis-placement of the ventricles, and cortical atrophy. Whole-body CT scanners
allow sections of the spinal cord to be visualized. The injection of a
water-soluble iodinated contrast agent into the subarachnoid space through
lumbar puncture improves the visu-alization of the spinal and intracranial
contents on these images. The CT scan, along with magnetic resonance imaging
(MRI), has largely replaced myelography as a diagnostic procedure for the
di-agnosis of herniated lumbar disks.
CT scanning is usually performed first without
contrast ma-terial and then with intravenous contrast enhancement. The pa-tient
lies on an adjustable table with the head held in a fixed position, while the
scanning system rotates around the head and produces cross-sectional images.
The patient must lie with the head held perfectly still without talking or
moving the face, be-cause head motion will distort the image.
CT scanning is noninvasive and painless and has a
high degree of sensitivity for detecting lesions. With advances in CT scanning,
the number of disorders and injuries that can be diagnosed is increasing.
Nursing Interventions
Essential
nursing interventions include preparation for the pro-cedure and patient
monitoring. Preparation includes teaching the patient about the need to lie
quietly throughout the procedure. A review of relaxation techniques may be
helpful for claustropho-bic patients.
Sedation
can be used if agitation, restlessness, or confusion will interfere with a
successful study (Hinkle, 1999a). Ongoing patient monitoring during sedation is
necessary. If a contrast agent is used, the patient must be assessed before the
CT scan for an iodine/shellfish allergy, as the contrast agent is iodine-based.
An intravenous line for injection of the contrast agent and a pe-riod of
fasting (usually 4 hours) are required prior to the study. Patients who receive
an intravenous or inhalation contrast agent are monitored during and after the
procedure for allergic re-actions and other side effects, including flushing,
nausea, and vomiting.
POSITRON EMISSION TOMOGRAPHY
Positron
emission tomography (PET) is a computer-based nu-clear imaging technique that
produces images of actual organ functioning. The patient either inhales a
radioactive gas or is in-jected with a radioactive substance that emits
positively charged particles. When these positrons combine with negatively
charged electrons (normally found in the body’s cells), the resultant gamma
rays can be detected by a scanning device that produces a series of two-dimensional
views at various levels of the brain. This information is integrated by a
computer and gives a composite picture of the brain at work.
PET
permits the measurement of blood flow, tissue composi-tion, and brain
metabolism and thus indirectly evaluates brain function. The brain is one of
the most metabolically active or-gans, consuming 80% of the glucose the body
uses. PET mea-sures this activity in specific areas of the brain and can detect
changes in glucose use.
This
test is useful in showing metabolic changes in the brain (Alzheimer’s disease),
locating lesions (brain tumor, epilepto-genic lesions), identifying blood flow
and oxygen metabolism in patients with strokes, evaluating new therapies for
brain tumors, and revealing biochemical abnormalities associated with mental
illness. The isotopes used have a very short half-life and are ex-pensive to
produce, requiring specialized equipment for pro-duction. PET scanning has been
useful in research settings for the last 20 years and is now becoming more
available in clinical settings. Improvements in scanning itself and the
production of isotopes, as well as the advent of reimbursement by third-party
payers, has increased the availability of PET studies (Gjedde et al., 2001).
Nursing Interventions
Key nursing interventions include patient
preparation, which in-volves explaining the test and teaching the patient about
inhala-tion techniques and the sensations (eg, dizziness, lightheadedness, and
headache) that may occur. The intravenous injection of the radioactive
substance produces similar side effects. Relaxation ex-ercises may reduce
anxiety during the test.
SINGLE PHOTON EMISSION COMPUTED TOMOGRAPHY
Single
photon emission computed tomography (SPECT) is a three-dimensional imaging
technique that uses radionuclides and instruments to detect single photons. It
is a perfusion study that captures a moment of cerebral blood flow at the time
of injection of a radionuclide (Huntington, 1999). Gamma photons are emit-ted
from a radiopharmaceutical agent administered to the patient and are detected
by a rotating gamma camera or cameras; the image is sent to a minicomputer.
This approach allows areas be-hind overlying structures or background to be
viewed, greatly in-creasing the contrast between normal and abnormal tissue. It
is relatively inexpensive, and the duration is similar to that of a CT scan.
SPECT
is useful in detecting the extent and location of ab-normally perfused areas of
the brain, thus allowing detection, lo-calization, and sizing of stroke (before
it is visible by CT scan), localization of seizure foci in epilepsy, and
evaluation of perfu-sion before and after neurosurgical procedures. Pregnancy
and breastfeeding are contraindications to SPECT.
Nursing Interventions
The
nursing interventions for SPECT primarily include patient preparation and
patient monitoring. Teaching about what to ex-pect before the test can allay
anxiety and ensure patient coopera-tion during the test. Premenopausal women
are advised to practice effective contraception before and for several days
after testing, and the woman who is breastfeeding is instructed to stop nursing
for the period of time recommended by the nuclear med-icine department.
The
nurse may need to accompany and monitor the patient during transport to the nuclear
medicine department for the scan. Patients are monitored during and after the
procedure for allergic reactions to the radiopharmaceutical agent. In a few
institutions nurses with special education and training inject the contrast
agent before a SPECT scan (Fischbach, 2002; Huntington, 1999).
MAGNETIC RESONANCE IMAGING
Magnetic resonance imaging (MRI) uses a powerful magnetic field to obtain images of different areas of the body (Fig. 60-16). This diagnostic test involves altering hydrogen ions in the body. Placing the patient into a powerful magnetic field causes the hydrogen nuclei (protons) within the body to align like small magnets in a magnetic field.
In combination with
radiofrequency pulses, the protons emit signals, which are converted to images.
MRI has the potential for identifying a cerebral abnormality earlier and more
clearly than other diagnostic tests. It can provide information about the
chemical changes within cells, allowing the clinician to monitor a tumor’s
response to treatment. It is partic-ularly useful in the diagnosis of multiple
sclerosis and can describe the activity and extent of disease in the brain and
spinal cord. MRI does not involve ionizing radiation.
Several newer MRI techniques, including magnetic
reso-nance angiography (MRA), diffusion-weighted imaging (DWI),
perfusion-weighted imaging (PWI), and fluid attenuation inver-sion recovery
(FLAIR), are becoming more widely used (Hinkle, 1999b; Shellock, 2001). The use
of MRA allows visualization of the cerebral vasculature without the
administration of an arterial contrast agent. A substantial amount of research
on the tech-niques of DWI, PWI, and FLAIR shows promise for clearer
visualization and the early diagnosis of ischemic stroke (Hinkle, 1999b). At
present MRI is most valuable in the diagnosis of nonacute conditions, as the
test takes up to an hour to complete.
Nursing Interventions
Patient
preparation should include teaching relaxation tech-niques and informing the
patient that he or she will be able to talk to the staff by means of a
microphone located inside the scanner. Many MRI suites provide headphones so
patients can listen to the music of their choice during the procedure.
Before the patient enters the room where the MRI is
to be per-formed, all metal objects and credit cards (the magnetic field can
erase them) are removed. No metal objects may be brought into the room where
the MRI is located (Shellock, 2001): this in-cludes oxygen tanks, traditional
ventilators, or even stethoscopes. The magnetic field generated by the unit is
so strong that any metal-containing items will be strongly attracted and
literally can be pulled away with such force that they fly like projectiles
toward the magnet. There is a risk of severe injury and death; further-more,
damage to a very expensive piece of equipment may occur. A patient history is
obtained to determine the presence of any metal objects (eg, aneurysm clips,
orthopedic hardware, pace-makers, artificial heart valves, intrauterine devices).
These objects could malfunction, be dislodged, or heat up as they absorb
en-ergy. Cochlear implants will be inactivated by MRI; therefore, other imaging
procedures are considered.
The
patient lies on a flat platform that is moved into a tube housing the magnet.
The scanning process is painless, but the pa-tient hears loud thumping of the
magnetic coils as the magnetic field is being pulsed. Because the MRI scanner
is a narrow tube, patients may experience claustrophobia; sedation may be
pre-scribed in these circumstances. Newer versions of MRI machines are less
claustrophobic than the earlier devices and are available in some locations.
However, the images produced on these ma-chines are not optimal, and the
traditional device is preferable for accurate diagnosis.
CEREBRAL ANGIOGRAPHY
Cerebral angiography is an x-ray study of the
cerebral circulation with a contrast agent injected into a selected artery.
Cerebral angiography is a valuable tool to investigate vascular disease,
aneurysms, and arteriovenous malformations. It is frequently per-formed before
craniotomy to assess the patency and adequacy of the cerebral circulation and
to determine the site, size, and nature of the pathologic processes (Fischbach,
2002; Frizzell, 1998).
Most
cerebral angiograms are performed by threading a catheter through the femoral
artery in the groin and up to the desired ves-sel. Alternatively, direct
puncture of the carotid or vertebral artery or retrograde injection of a
contrast agent into the brachial artery may be performed.
In digital subtraction angiography (DSA), x-ray
images of the area in question are obtained before and after the injection of a
contrast agent. The computer analyzes the differences between the two images
and produces an enhanced image of the carotid and vertebral arterial systems.
The injection for a DSA can be given through a peripheral vein (Fischbach,
2002; Rowland, 2000).
Nursing Interventions
The
patient should be well hydrated, and clear liquids are usually permitted up to
the time of a regular arteriogram or DSA. Before going to the x-ray department,
the patient is instructed to void. The locations of the appropriate peripheral
pulses are marked with a felt-tip pen. The patient is instructed to remain
immobile during the angiogram process and is told to expect a brief feel-ing of
warmth in the face, behind the eyes, or in the jaw, teeth, tongue, and lips,
and a metallic taste when the contrast agent is injected.
After
the groin is shaved and prepared, a local anesthetic is ad-ministered to prevent
pain at the insertion site and to reduce ar-terial spasm. A catheter is
introduced into the femoral artery, flushed with heparinized saline, and filled
with contrast agent. Fluoroscopy is used to guide the catheter to the
appropriate ves-sels. During injection of the contrast agent, images are made
of the arterial and venous phases of circulation through the brain.
Nursing care after cerebral angiography includes
observation for signs and symptoms of altered cerebral blood flow. In some
instances, patients may experience major or minor arterial block-age due to
embolism, thrombosis, or hemorrhage, producing a neurologic deficit. Signs of
such an occurrence include alterations in the level of responsiveness and
consciousness, weakness on one side of the body, motor or sensory deficits, and
speech distur-bances. Therefore, it is necessary to observe the patient
frequently for these signs and to report them immediately if they occur.
The injection site is observed for hematoma
formation (a lo-calized collection of blood), and an ice bag may be applied
inter-mittently to the puncture site to relieve swelling and discomfort.
Because a hematoma at the puncture site or embolization to a dis-tant artery
affects the peripheral pulses, these pulses are moni-tored frequently. The
color and temperature of the involved extremity are assessed to detect possible
embolism.
MYELOGRAPHY
A myelogram
is an x-ray of the spinal subarachnoid space taken after the injection of a
contrast agent into the spinal subarachnoid space through a lumbar puncture. It
outlines the spinal sub-arachnoid space and shows any distortion of the spinal
cord or spinal dural sac caused by tumors, cysts, herniated vertebral disks,or
other lesions. Water-based agents have replaced oil-based agents and their use
has reduced side effects and complications; these agents disperse upward
through the CSF. Myelography is performed less frequently today because of the
sensitivity of CT scanning and MRI (Hickey, 2003).
Nursing Interventions
Because
many patients have misconceptions about this proce-dure, the nurse clarifies
the explanation given by the physician and answers questions. The patient is
informed about what to ex-pect during the procedure and should be aware that
changes in position may be made during the procedure. The meal that nor-mally
would be eaten before the procedure is omitted. A sedative may be prescribed to
help the patient cope with this rather lengthy test. Patient preparation for
lumbar puncture is discussed later.
After myelography, the patient lies in bed with the
head of the bed elevated 30 to 45 degrees. The patient is advised to remain in
bed in the recommended position for 3 hours or as prescribed by the physician.
The patient is encouraged to drink liberal amounts of fluid for rehydration and
replacement of CSF and to decrease the incidence of postlumbar puncture
headache. The blood pressure, pulse, respiratory rate, and temperature are
mon-itored, as well as the patient’s ability to void. Untoward signs in-clude
headache, fever, stiff neck, photophobia
(sensitivity to light), seizures, and signs of chemical or bacterial
meningitis.
NONINVASIVE CAROTID FLOW STUDIES
Noninvasive carotid flow studies use ultrasound
imagery and Doppler measurements of arterial blood flow to evaluate carotid and
deep orbital circulation. The graph produced indicates blood ve-locity.
Increased blood velocity can indicate stenosis or partial ob-struction. These
tests are often obtained before arteriography, which carries a higher risk of
stroke or death (Fischbach, 2002; Hickey, 2003). Carotid Doppler, carotid
ultrasonography, oculo-plethysmography, and ophthalmodynamometry are four
common noninvasive vascular techniques that permit evaluation of arterial blood
flow and detection of arterial stenosis, occlusion, and plaques.
TRANSCRANIAL DOPPLER
Transcranial
Doppler uses the same noninvasive techniques as carotid flow studies except
that it records the blood flow veloci-ties of the intracranial vessels. Flow
velocities of the basal artery can be measured through thin areas of the
temporal and occipi-tal bones of the skull. A hand-held Doppler probe emits a
pulsed beam; the signal is reflected by the moving red blood cells within the
blood vessels (Falyar, 1999). Transcranial Doppler sonogra-phy is a noninvasive
technique that is helpful in assessing va-sospasm (a complication following
subarachnoid hemorrhage), altered cerebral blood flow found in occlusive
vascular disease or stroke, and other cerebral pathology.
Nursing Interventions
When
a carotid flow study or transcranial Doppler is scheduled, the procedure is
described to the patient. The patient is informed that this is a noninvasive
test, that a hand-held transducer will be placed over the neck and orbits of
the eyes, and that some type of water-soluble jelly is used on the transducer.
Either one of these low-risk tests can be performed at the patient’s bedside.
ELECTROENCEPHALOGRAPHY
An
electroencephalogram (EEG) represents a record of the elec-trical activity
generated in the brain. It is obtained through elec-trodes applied on the scalp
or through microelectrodes placed within the brain tissue. It provides a
physiologic assessment of cerebral activity.
The
EEG is a useful test for diagnosing and evaluating seizure disorders, coma, or
organic brain syndrome. Tumors, brain ab-scesses, blood clots, and infection
may cause abnormal patterns in electrical activity. The EEG is also used in
making a determi-nation of brain death.
Electrodes
are applied to the scalp to record the electrical ac-tivity in various regions
of the brain. The amplified activity of the neurons between any two of these
electrodes is recorded on con-tinuously moving paper; this record is called the
encephalogram.
For
a baseline recording, the patient lies quietly with both eyes closed. The
patient may be asked to hyperventilate for 3 to 4 min-utes and then look at a
bright, flashing light for photic stimulation. These activation procedures are
performed to evoke abnormal elec-trical discharges, such as seizure potentials.
A sleep EEG may be recorded after sedation because some abnormal brain waves
are seen only when the patient is asleep. If the epileptogenic area is
inaccessible to conventional scalp electrodes, nasopharyngeal elec-trodes may
be used.
Depth recording of EEG is performed by introducing
elec-trodes stereotactically (radiologically placed using instrumentation) into
a target area of the brain, as indicated by the patient’s seizure pattern and
scalp EEG. It is used to identify patients who may benefit from surgical
excision of epileptogenic foci.
Special
transsphenoidal, mandibular, and nasopharyngeal elec-trodes can be used, and
video recording combined with EEG monitoring and telemetry is used in hospital
settings to capture epileptiform abnormalities and their sequelae. Some
epilepsy cen-ters provide long-term ambulatory EEG monitoring with portable
recording devices.
Nursing Interventions
To increase the chances of recording seizure
activity, it is some-times recommended that the patient be deprived of sleep on
the night before the EEG. Antiseizure agents, tranquilizers, stimu-lants, and
depressants should be withheld 24 to 48 hours before an EEG because these
medications can alter the EEG wave pat-terns or mask the abnormal wave patterns
of seizure disorders (Hickey, 2003). Coffee, tea, chocolate, and cola drinks
are omit-ted in the meal before the test because of their stimulating effect.
The meal is not omitted, however, because an altered blood glu-cose level can
also cause changes in the brain wave patterns.
The
patient is informed that the standard EEG takes 45 to 60 minutes, 12 hours for
a sleep EEG. The patient is assured that the procedure does not cause an
electric shock and that the EEG is a diagnostic test, not a form of treatment.
An EEG requires pa-tient cooperation and ability to lie quietly during the
test. Seda-tion is not advisable as it may lower the seizure threshold in
patients with a seizure disorder and alter brain wave activity in all patients.
Patients with seizures do not stop taking their anti-seizure medication prior
to testing.
Routine
EEGs use a water-soluble lubricant for electrode con-tact, which at the
conclusion of the study can be wiped off and removed by shampooing. Sleep EEGs
involve the use of col-lodion glue for electrode contact, which requires
acetone for removal.
EVOKED POTENTIAL STUDIES
In evoked potential studies, electrodes are applied
to the scalp and an external stimulus is applied to peripheral sensory
receptors to elicit changes in the brain waves. Evoked changes are detected with
the aid of computerized devices that extract the signal, dis-play it on an
oscilloscope, and store the data on magnetic tape or disk. These studies are
based on the concept that any insult or dys-function that can alter neuronal
metabolism or disturb membrane function may change evoked responses in brain
waves. In neuro-logic diagnosis, they reflect conduction times in the
peripheral nervous system. In clinical practice, the visual, auditory, and
somatosensory systems are most often tested.
In visual evoked responses, the patient looks at a
visual stimu-lus (flashing lights, a checkerboard pattern on a screen). The
av-erage of several hundred stimuli is recorded by EEG leads placed over the
occiput. The transit time from the retina to the occipital area is measured
using computer-averaging methods.
Auditory
evoked responses or brain stem evoked responses are measured by applying an
auditory stimulus (a repetitive auditory click) and measuring the transit time
up the brain stem into the cortex. Specific lesions in the auditory pathway
modify or delay the response.
In
somatosensory evoked responses, the peripheral nerves are stimulated
(electrical stimulation through skin electrodes) and the transit time up the
spinal cord to the cortex is measured and recorded from scalp electrodes.
This
test is used to detect a deficit in spinal cord conduction and to monitor
spinal cord function during operative procedures. Because myelinated fibers
conduct impulses at a higher rate of speed, nerves with an intact myelin sheath
record the highest ve-locity. Demyelination of nerve fibers leads to a decrease
in speed of conduction, as found in Guillain-Barré syndrome, multiple
sclerosis, and polyneuropathies.
Nursing Interventions
There is no specific patient preparation other than
to explain the procedure and to reassure the patient and encourage him or her
to relax. The patient is advised to remain perfectly still throughout the
recording to prevent artifacts (signals not generated by the brain) that
interfere with the recording and interpretation of the test.
ELECTROMYOGRAPHY
An electromyogram (EMG) is obtained by introducing
needle electrodes into the skeletal muscles to measure changes in the
electrical potential of the muscles and the nerves leading to them. The
electrical potentials are shown on an oscilloscope and am-plified by a
loudspeaker so that both the sound and appearance of the waves can be analyzed
and compared simultaneously.
An
EMG is useful in determining the presence of a neuro-muscular disorder and
myopathies. They help to distinguish weakness due to neuropathy (functional or
pathologic changes in the peripheral nervous system) from weakness due to other
causes.
Nursing Interventions
The
procedure is explained and the patient is warned to expect a sensation similar
to that of an intramuscular injection as the nee-dle is inserted into the
muscle. The muscles examined may ache for a short time after the procedure.
NERVE CONDUCTION STUDIES
Nerve
conduction studies are performed by stimulating a pe-ripheral nerve at several
points along its course and recording the muscle action potential or the
sensory action potential that re-sults. Surface or needle electrodes are placed
on the skin over the nerve to stimulate the nerve fibers. This test is useful
in the study of peripheral neuropathies.
LUMBAR PUNCTURE AND EXAMINATION OF CEREBROSPINAL FLUID
A
lumbar puncture (spinal tap) is carried out by inserting a nee-dle into the
lumbar subarachnoid space to withdraw CSF. The test may be performed to obtain
CSF for examination, to mea-sure and reduce CSF pressure, to determine the
presence or ab-sence of blood in the CSF, to detect spinal subarachnoid block,
and to administer antibiotics intrathecally (into the spinal canal) in certain
cases of infection.
The
needle is usually inserted into the subarachnoid space be-tween the third and
fourth or fourth and fifth lumbar vertebrae. Because the spinal cord divides
into a sheaf of nerves at the first lumbar vertebra, insertion of the needle
below the level of the third lumbar vertebra prevents puncture of the spinal
cord.
A
successful lumbar puncture requires that the patient be re-laxed; an anxious
patient is tense, and this may increase the pres-sure reading. CSF pressure
with the patient in a lateral recumbent position is normally 70 to 200 mm H2O.
Pressures of more than 200 mm H2O
are considered abnormal.
A
lumbar puncture may be risky in the presence of an in-tracranial mass lesion
because intracranial pressure is decreased by the removal of CSF, and the brain
may herniate downward through the tentorium and the foramen magnum.
Queckenstedt’s Test
A lumbar manometric test (Queckenstedt’s test) may
be per-formed by compressing the jugular veins on each side of the neck during
the lumbar puncture. The increase in pressure caused by the compression is
noted; then the pressure is released and pres-sure readings are made at
10-second intervals. Normally, CSF pressure rises rapidly in response to
compression of the jugular veins and returns quickly to normal when the
compression is re-leased. A slow rise and fall in pressure indicates a partial
block due to a lesion compressing the spinal subarachnoid pathways. If there is
no pressure change, a complete block is indicated. This test is not performed
if an intracranial lesion is suspected.
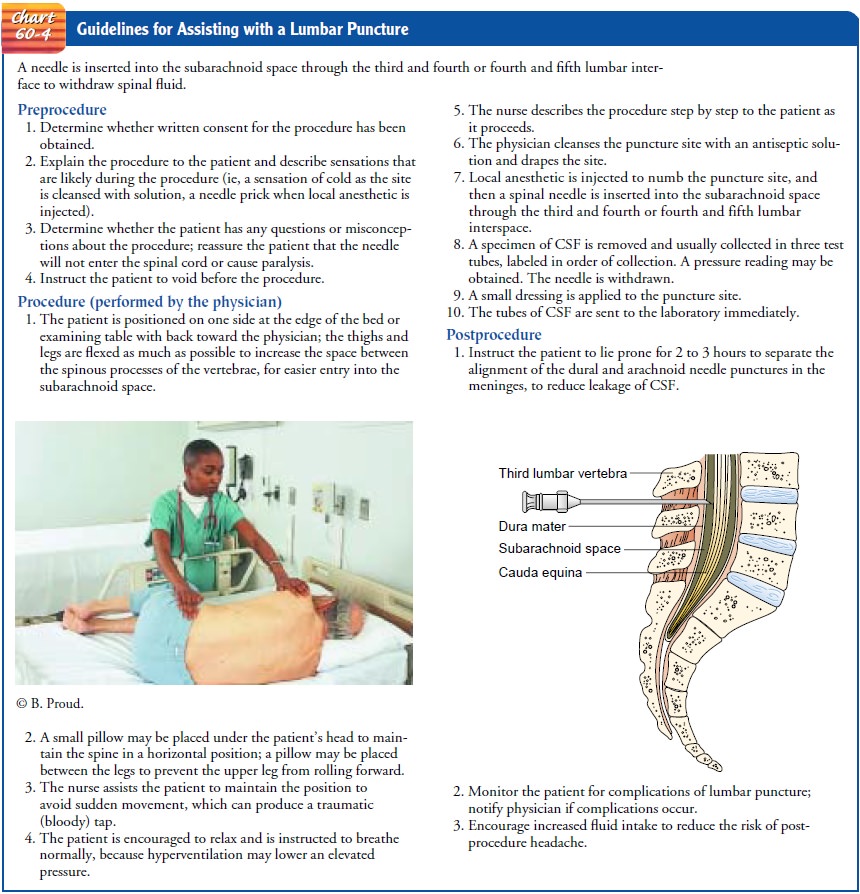
See
Chart 60-4 for nursing guidelines for assisting with a lum-bar puncture.
Cerebrospinal Fluid Analysis
The
CSF should be clear and colorless. Pink, blood-tinged, or grossly bloody CSF
may indicate a cerebral contusion, laceration, or subarachnoid hemorrhage.
Sometimes with a difficult lumbar puncture, the CSF initially is bloody because
of local trauma but then becomes clearer.
Usually, specimens are obtained for cell count,
culture, and glucose and protein testing. The specimens should be sent to the
laboratory immediately because changes will take place and alterthe
result if the specimens are allowed to stand. (See Appendix B for the normal
values of CSF.)
Post–Lumbar Puncture Headache
A
post–lumbar puncture headache, ranging from mild to severe, may appear a few
hours to several days after the procedure. This is the most common
complication, occurring in 15% to 30% of patients (Connolly, 1999). It is a
throbbing bifrontal or occipital headache, dull and deep in character. It is
particularly severe on sitting or standing but lessens or disappears when the
patient lies down.
The
headache is caused by CSF leakage at the puncture site. The fluid continues to
escape into the tissues by way of the needle track from the spinal canal. It is
then absorbed promptly by the lymphatics. As a result of this leak, the supply
of CSF in the cranium is depleted to a point at which it is insufficient to
maintain proper mechanical stabilization of the brain. This leakage of CSF
allows settling of the brain when the patient as-sumes an upright position,
producing tension and stretching the venous sinuses and pain-sensitive
structures. Both traction and pain are lessened and the leakage is reduced when
the pa-tient lies down.
Post–lumbar
puncture headache may be avoided if a small-gauge needle is used and if the
patient remains prone after the procedure. When a large volume of fluid (more
than 20 mL) is removed, the patient is positioned prone for 2 hours, then flat
in a side-lying position for 2 to 3 hours, and then supine or prone for 6 more
hours. Keeping the patient flat overnight may reduce the incidence of
headaches.
The
postpuncture headache is usually managed by bed rest, analgesic agents, and
hydration (Connolly, 1999). Occasionally, if the headache persists, the
epidural blood patch technique may be used. Blood is withdrawn from the
antecubital vein and in-jected into the epidural space, usually at the site of
the previous spinal puncture. The rationale is that the blood acts as a
gelati-nous plug to seal the hole in the dura, preventing further loss of CSF.
Other Complications of Lumbar Puncture
Herniation
of the intracranial contents, spinal epidural abscess, spinal epidural
hematoma, and meningitis are rare but serious complications of lumbar puncture.
Other complications include temporary voiding problems, slight elevation of
temperature, backache or spasms, and stiffness of the neck.
HOME AND COMMUNITY-BASED CARE
Teaching Patients Self-Care
Many diagnostic tests that were once performed as part of a hos-pital stay are now carried out in short-procedure units or out-patient testing settings or units. As a result, family members often provide the postprocedure care. Therefore, the patient and family must receive clear verbal and written instructions about precautions to take after the procedure, complications to watch for, and steps to take if complications occur. Because many pa-tients undergoing neurologic diagnostic studies are elderly or have neurologic deficits, provisions must be made to ensure that transportation and postprocedure care and monitoring are avail-able.
Continuing Care
Contacting
the patient and family after diagnostic testing enables the nurse to determine
whether they have any questions about the procedure or whether the patient had
any untoward results. During these phone calls, teaching is reinforced and the
patient and fam-ily are reminded to make and keep follow-up appointments.
Patients, family members, and health care providers are focused on the immediate
needs, issues, or deficits that necessitated the diagnostic testing. This is
also a good time to remind them of the need for and importance of continuing
health promotion and screening practices and make referrals to appropriate
health care providers.
Related Topics