Chapter: Pharmaceutical Biotechnology: Fundamentals and Applications : Production and Downstream Processing of Biotech Compounds
Cultivation Medium - Production of Biotech Compounds
Cultivation
Medium
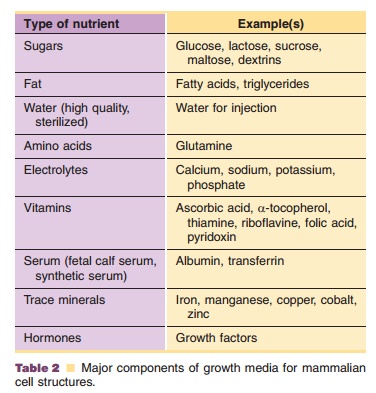
In order to achieve optimal growth of cells it is of great importance that not only conditions such as pH, oxygen tension and temperature are chosen and mixture of diverse components, such as sugars, amino acids, electrolytes, vitamins, fetal calf serum and a mixture of peptones, growth factors, hormones, and other proteins (Table 2). Many of these ingredients are pre-blended either diluted or as homogeneous mixtures of pow-ders. To prepare the final medium, components are dissolved in purified water before sterile filtration. Some supplements, especially fetal calf serum, con-tribute considerably to the presence of contaminating proteins and may seriously complicate purification procedures. Additionally, the composition of serum is variable; it depends on the individual animal, season of the year, suppliers’ treatment, etc. The use of serum may introduce adventitious material such as viruses, mycoplasmas, bacteria and fungi into the culture system (Berthold and Walter, 1994). Furthermore, the possible presence of prions that can cause transmis-sible spongiform encephalitis (TSE) almost precludes the use bovine, sheep or goat material. However, if use of this material is inevitable, one must follow the relevant guidelines in which selective sourcing of the material is still the key measure to safety (EMEA, 1999a). Many of these potential problems when using serum in cell culture media have resulted in the creation of serum-free formulations, particularly by the medium suppliers, and a trend toward the removal of other complex undefined medium components. Completely serum-free and chemically defined media have been shown to give satisfactory results in industrial scale production settings in certain cases, for example, in monoclonal antibody production.
Related Topics