Chapter: Basic & Clinical Pharmacology : Drugs Used in Heart Failure
Control of Normal Cardiac Contractility
Control of Normal Cardiac
Contractility
The
vigor of contraction of heart muscle is determined by several processes that
lead to the movement of actin and myosin filaments in the cardiac sarcomere
(Figure 13–1). Ultimately, contraction results from the interaction of activator calcium (during systole) with
the actin-troponin-tropomyosin system, thereby releasing the actin-myosin
interaction. This activator calcium is released from the sarcoplasmic reticulum
(SR). The amount released depends on the amount stored in the SR and on the
amount of trigger calcium that enters
the cell during the plateau of the action potential.
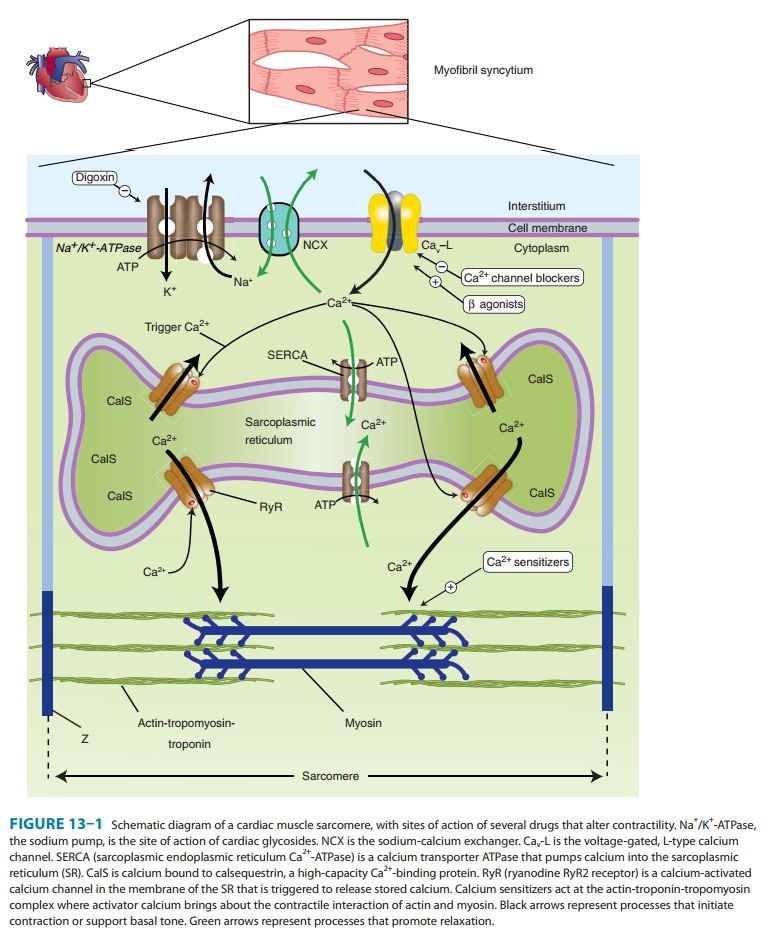
A. Sensitivity of the Contractile Proteins to Calcium and Other Contractile Protein Modifications
The
determinants of calcium sensitivity, ie, the curve relating the shortening of
cardiac myofibrils to the cytoplasmic calcium con-centration, are incompletely
understood, but several types of drugs can be shown to affect calcium
sensitivity in vitro. Levosimendan
is the most recent example of a drug that increases calcium sensi-tivity (it
may also inhibit phosphodiesterase) and reduces symp-toms in models of heart
failure.
A
recent report suggests that an experimental drug, omecantivmecarbil (CK-1827452), alters the rate of transition of
myosinfrom a low-actin-binding state to a strongly actin-bound force-generating
state. Preliminary studies in experimental animal mod-els of heart failure
indicate that this agent may provide a new approach to the treatment of heart
failure in humans. Clinical tri-als are underway.
B. Amount of Calcium Released from the Sarcoplasmic Reticulum
A
small rise in free cytoplasmic calcium, brought about by calcium influx during
the action potential, triggers the opening of calcium-gated,
ryanodine-sensitive calcium channels (RyR2) in the mem-brane of the cardiac SR
and the rapid release of a large amount of the ion into the cytoplasm in the
vicinity of the actin-troponin-tropomyosin complex. The amount released is
proportional to the amount stored in the SR and the amount of trigger calcium
thatenters the cell through the cell membrane. (Ryanodine is a potent negative
inotropic plant alkaloid that interferes with the release of calcium through cardiac
SR channels.)
C. Amount of Calcium Stored in the Sarcoplasmic Reticulum
The
SR membrane contains a very efficient calcium uptake trans-porter known as the
sarcoplasmic endoplasmic reticulum Ca2+-ATPase (SERCA). This
pump maintains free cytoplasmic calcium at very low levels during diastole by
pumping calcium into the SR. SERCA is normally inhibited by phospholamban;
phosphoryla-tion of phospholamban by protein kinase A (eg, by β agonists) removes
this inhibition. The amount of calcium sequestered in the SR is thus
determined, in part, by the amount accessible to this transporter and the
activity of the sympathetic nervous system. This in turn is dependent on the
balance of calcium influx (pri-marily through the voltage-gated membrane L-type
calcium chan-nels) and calcium efflux, the amount removed from the cell
(primarily via the sodium-calcium exchanger, a transporter in the cell
membrane). The amount of Ca2+ released from the SR
depends on the response of the RyR channels to trigger Ca2+.
D. Amount of Trigger Calcium
The
amount of trigger calcium that enters the cell depends on the availability of
membrane calcium channels and the duration of their opening. As described,
sympathomimet-ics cause an increase in calcium influx through an action on
these channels. Conversely, the calcium channel blockers reduce this influx and depress contractility.
E. Activity of the Sodium-Calcium Exchanger
This
antiporter (NCX) uses the sodium gradient to move calcium against its
concentration gradient from the cytoplasm to the extra-cellular space.
Extracellular concentrations of these ions are much less labile than
intracellular concentrations under physiologic con-ditions. The sodium-calcium
exchanger’s ability to carry out this transport is thus strongly dependent on
the intracellular concen-trations of both ions, especially sodium.
F. Intracellular Sodium Concentration and Activity of Na+/K+-ATPase
Na+/K+-ATPase, by removing
intracellular sodium, is the major determinant of sodium concentration in the
cell. The sodium influx through voltage-gated channels, which occurs as a
normal part of almost all cardiac action potentials, is another determinant,
although the amount of sodium that enters with each action potential is much
less than 1% of the total intracellular sodium. Na+/K+-ATPase appears to be
the primary target of digoxin and
other cardiac glycosides.
Related Topics