Chapter: Basic & Clinical Pharmacology : Drugs Used in Heart Failure
Basic Pharmacology of Drugs Used in Heart Failure
BASIC
PHARMACOLOGY OF DRUGS USED IN HEART FAILURE
Although
digitalis is not the first drug and never the only drug used in heart failure.
DIGITALIS
Digitalis
is the genus name for the family of plants that provide most of the medically
useful cardiac glycosides, eg,
digoxin. Such plants have been known for thousands of years but were used
erratically and with variable success until 1785, when William Withering, an
English physician and botanist, published a mono-graph describing the clinical
effects of an extract of the purple foxglove plant (Digitalis purpurea, a major source of these agents).
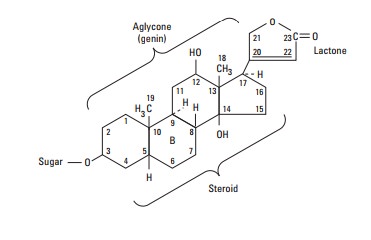
Chemistry
All of the cardiac glycosides, or cardenolides—of which digoxin is the prototype—combine a
steroid nucleus linked to a lactone ring at the 17 position and a series of
sugars at carbon 3 of the nucleus. Because they lack an easily ionizable group,
their solubility is not pH-dependent. Digoxin is obtained from Digitalis lanata, the white foxglove,
but many common plants (eg, oleander, lily of the valley, and milkweed) contain
cardiac glycosides with similar properties.
Pharmacokinetics
Digoxin,
the only cardiac glycoside used in the USA, is 65–80% absorbed after oral
administration. Absorption of other glycosides varies from zero to nearly 100%.
Once present in the blood, all cardiac glycosides are widely distributed to
tissues, including the central nervous system.
Digoxin
is not extensively metabolized in humans; almost two thirds is excreted
unchanged by the kidneys. Its renal clearance is proportional to creatinine
clearance, and the half-life is 36–40 hours in patients with normal renal
function. Equations and nomograms are available for adjusting digoxin dosage in
patients with renal impairment.
Pharmacodynamics
Digoxin
has multiple direct and indirect cardiovascular effects, with both therapeutic
and toxic consequences. In addition, it has undesirable effects on the central
nervous system and gut.At the molecular level, all therapeutically useful
cardiac glyco-sides inhibit Na+/K+-ATPase, the membrane-bound transporter often called the sodium pump (Figure 13–1). Although
several isoforms of this ATPase occur and have varying sensitivity to car-diac
glycosides, they are highly conserved in evolution. Inhibition of this
transporter over most of the dose range has been extensively documented in all
tissues studied. It is probable that this inhibi-tory action is largely
responsible for the therapeutic effect (positive inotropy) as well as a major
portion of the toxicity of digitalis. Other molecular-level effects of
digitalis have been studied in theheart and are discussed below. The fact that
a receptor for cardiac glycosides exists on the sodium pump has prompted some
investi-gators to propose that an endogenous digitalis-like steroid, possi-bly ouabain or marinobufagenin, must exist. Furthermore, additional functions of
Na+/K+-ATPase have been
postulated, involving apoptosis, cell growth and differentiation, immunity, and
carbohydrate metabolism.
A. Cardiac Effects
1. Mechanical effects— Cardiac glycosides increase contrac-tion of the cardiac sarcomere by increasing the free calcium con-centration in the vicinity of the contractile proteins during systole. The increase in calcium concentration is the result of a two-step process: first, an increase of intracellular sodium concentration because of Na+/K+-ATPase inhibition; and second, a relative reduction of calcium expulsion from the cell by the sodium-calcium exchanger (NCX in Figure 13–1) caused by the increase in intracellular sodium. The increased cytoplasmic calcium is sequestered by SERCA in the SR for later release. Other mecha-nisms have been proposed but are not well supported.
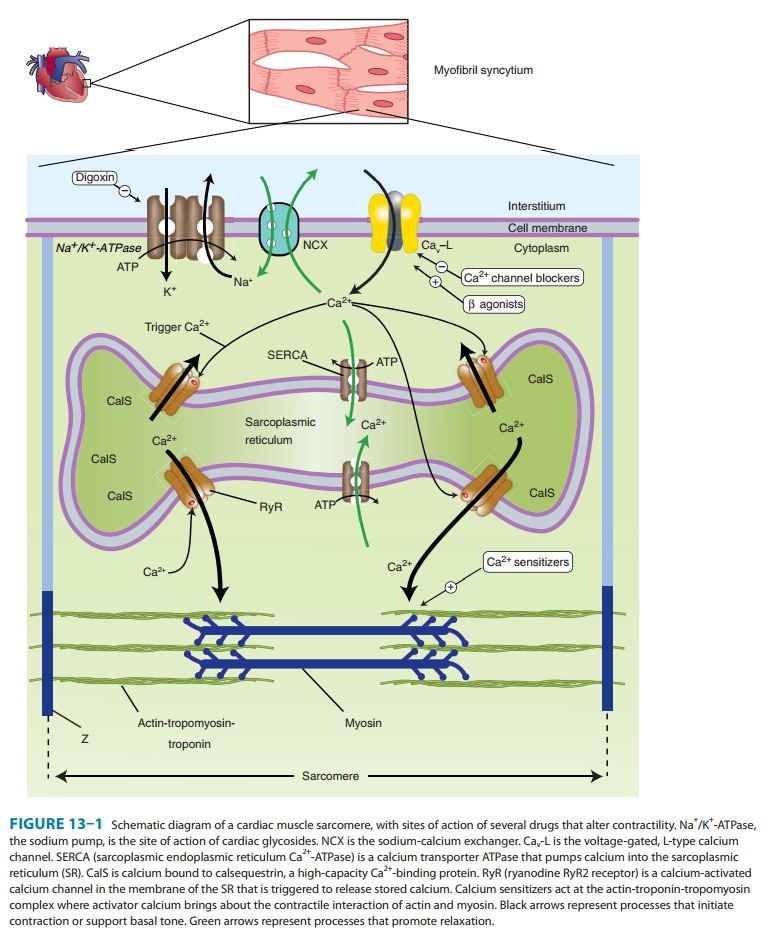
The
net result of the action of therapeutic concentrations of a cardiac glycoside
is a distinctive increase in cardiac contractility (Figure 13–5, bottom trace,
panels A and B). In isolated myocar-dial preparations, the rate of development
of tension and of relax-ation are both increased, with little or no change in
time to peak tension. This effect occurs in both normal and failing
myocar-dium, but in the intact patient the responses are modified by
cardiovascular reflexes and the pathophysiology of heart failure.
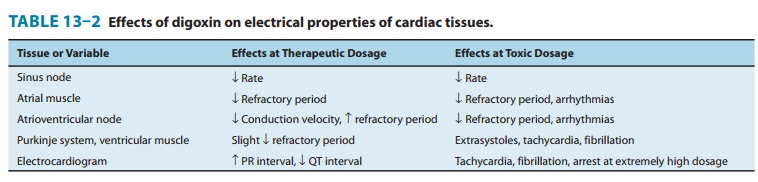
2. Electrical effects—The effects of
digitalis on the electricalproperties of the heart are a mixture of direct and
autonomic actions. Direct actions on the membranes of cardiac cells follow a
well-defined progression: an early, brief prolongation of the action potential,
followed by shortening (especially the plateau phase). The decrease in action
potential duration is probably the result of increased potassium conductance
that is caused by increased intra-cellular calcium . All these effects can be
observed at therapeutic concentrations in the absence of overt toxicity (Table
13–2).
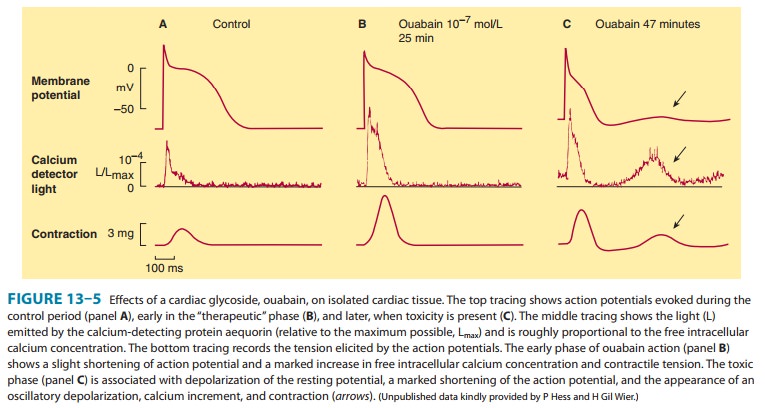
At higher concentrations, resting membrane potential is reduced
(made less negative) as a result of inhibition of the sodium pump and reduced
intracellular potassium. As toxicity progresses, oscillatory depolarizing
afterpotentials appear follow-ing normally evoked action potentials (Figure
13–5, panel C). The afterpotentials (also known as delayed after-depolariza-tions, DADs) are associated with
overloading of the intracellularcalcium stores and oscillations in the free
intracellular calcium ion concentration. When afterpotentials reach threshold,
they elicit action potentials (premature
depolarizations, ectopic “beats”) that are coupled to the preceding normal
action poten-tials. If afterpotentials in the Purkinje conducting system
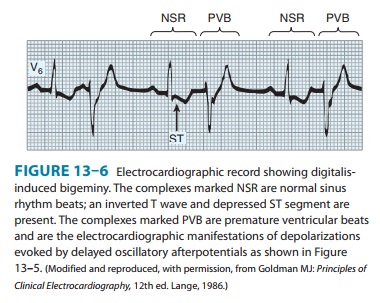
regu-larly reach threshold in this way, bigeminy will be recorded on the
electrocardiogram (Figure 13–6 ). With further intoxication, each
afterpotential-evoked action potential will itself elicit a suprathreshold
after-potential, and a self-sustaining tachycardia will be established. If
allowed to progress, such a tachycardia may deteriorate into fibrillation; in
the case of ventricular fibrillation, the arrhythmia will be rapidly fatal
unless corrected. Autonomic actions of cardiac glycosides on the heart involve
both the parasympathetic and the sympathetic systems. In the lower portion of
the dose range, cardioselective parasympathomi-metic effects predominate. In
fact, these atropine-blockable effects account for a significant portion of the
early electrical effects of digitalis (Table 13–2). This action involves
sensitization of the baroreceptors, central vagal stimulation, and facilitation
of musca-rinic transmission at the cardiac muscle cell. Because cholinergic
innervation is much richer in the atria, these actions affect atrial and
atrioventricular nodal function more than Purkinje or ven-tricular function.
Some of the cholinomimetic effects are useful in the treatment of certain
arrhythmias. At toxic levels, sympathetic outflow is increased by digitalis.
This effect is not essential for typical digitalis toxicity but sensitizes the
myocardium and exag-gerates all the toxic effects of the drug.
The most common cardiac manifestations of digitalis toxicity
include atrioventricular junctional rhythm, premature ventricular
depolarizations, bigeminal rhythm, and second-degree atrioven-tricular
blockade. However, it is claimed that digitalis can cause virtually any
arrhythmia.
B. Effects on Other Organs
Cardiac glycosides affect all excitable tissues, including
smooth muscle and the central nervous system. The gastrointestinal tract is the
most common site of digitalis toxicity outside the heart. The effects include
anorexia, nausea, vomiting, and diarrhea. This toxicity is caused in part by
direct effects on the gastrointestinal tract and in part by central nervous
system
Central nervous system effects include vagal and chemoreceptor trigger zone stimulation. Less often, disorientation and hallucinations— especially in the elderly—and visual disturbances are noted. The latter effect may include aberrations of color perception. Gynecomastia is a rare effect reported in men taking digitals.
C. Interactions with Potassium, Calcium, and Magnesium
Potassium
and digitalis interact in two ways. First, they inhibit each other’s binding to
Na+/K+-ATPase; therefore,
hyperkalemia reduces the enzyme-inhibiting actions of cardiac glycosides,
whereas hypokalemia facilitates these actions. Second, abnormal cardiac
automaticity is inhibited by hyperkalemia . Moderately increased extracellular
K+ therefore reduces the
effects of digitalis, especially the toxic effects.
Calcium
ion facilitates the toxic actions of cardiac glycosides by accelerating the
overloading of intracellular calcium stores that appears to be responsible for
digitalis-induced abnormal automa-ticity. Hypercalcemia therefore increases the
risk of a digitalis-in-duced arrhythmia. The effects of magnesium ion are
opposite to those of calcium. These interactions mandate careful evaluation of
serum electrolytes in patients with digitalis-induced arrhythmias.
Related Topics