Electricity | Term 2 Unit 2 | 7th Science - Conductors and Insulators | 7th Science : Term 2 Unit 2 : Electricity
Chapter: 7th Science : Term 2 Unit 2 : Electricity
Conductors and Insulators
Conductors and
Insulators
Based on the property of conductance of
electricity, substances are classified into two types, namely, Conductors and
Insulators (or) bad conductors of electricity.
The electrons of different types of atoms have different degrees of freedom to move around. With some types of materials, such as metals, the outermost electrons in the atoms are loosely bound and they chaotically move in the space between the atoms of that material. Because these virtually unbound electrons are free to leave their respective atoms and float around in the space between adjacent atoms, they are often called as free electrons.
Let’s imagine that we have a metal in the form of
a wire. When a voltage is connected across the ends of the metal wire, the free
electrons drift in one direction.
So, a really good conductor is one that has lots
of free charges while those who don’t have enough ‘free charges’ would not be
good at conducting electricity or we can say that they would be ‘poor
conductors’ of electricity.
Conductors
Conductors are the materials whose atoms have
electrons that are loosely bound and are free to move through the material. A
material that is a good conductor gives very little resistance to the flow of
charge (electron) on the application of external voltage. This flow of charge
(electron) is what constitutes an electric current. A good conductor has high
electrical conductivity in the above activity.
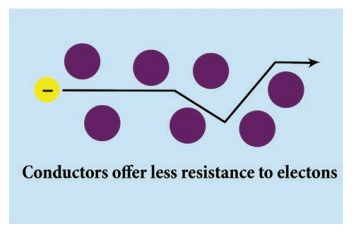
In general, more the free electrons, the better
the material will conduct (for a certain applied voltage).
Insulators
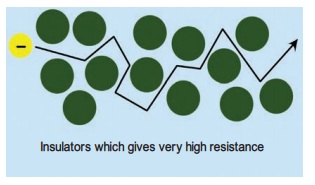
Those materials
which don’t have enough ‘free electrons’ are not good at conducting electricity
or we can say that they would be ‘poor conductors’ of electricity and they are
called insulators.
This is the material used in SIM
Cards, Computers, and ATM cards. Do you know by which material I am made up
off?
The chip which are used in SIM Cards, Computers, and
ATM cards are made up of semiconductors namely, silicon and germanium because
of their electrical conductivity lies between a conductor and an insulator.
An insulator gives a lot of resistance to the flow
of charge (electron). During the drift of the electrons in an object when an
external voltage is applied, collisions occur between the free electrons and
the atoms of the material also affect the movement of charges. These collisions
mean that they get scattered. It is a combination of the number of free
electrons and how much they are scattered that affects how well the metal
conducts electricity. The rubber eraser does not allow electric current to pass
through it. So rubber is a non-conductor of electricity. Rubber is an insulator
Most of the metals are good conductors of
electricity while most of the non-metals are poor conductors of electricity.
Wires made of copper, an nelectrical
conductor, have very low resistance. Copper wires are used to carry current in
households. These wires are in turn enclosed in electrical insulators, or
materials of high electrical resistance. These materials are usually made of
flexible plastic.

Related Topics