Chapter: Clinical Anesthesiology: Anesthetic Equipment & Monitors : Breathing Systems
Components of the Circle System
Components
of the Circle System
A. Carbon Dioxide Absorber and the Absorbent
Rebreathing
alveolar gas conserves heat and humid-ity. However, the CO2 in
exhaled gas must be elimi-nated to prevent hypercapnia. CO 2
chemically
combines
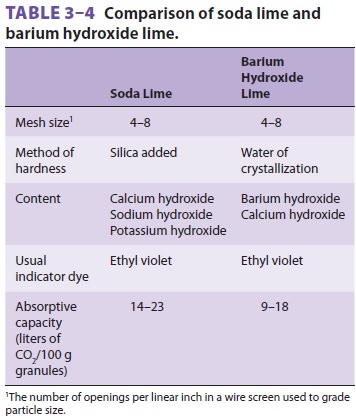
with water to form carbonic acid. CO2 absorbents (eg, soda lime or
calcium hydroxide lime) contain hydroxide salts that are capable of
neutralizing carbonic acid ( Table 3–4). Reaction end products include heat
(the heat of neutraliza-tion), water, and calcium carbonate. Soda lime is the more common absorbent
and is capable of absorb-ing up to 23 L of CO 2 per 100 g of
absorbent. It con-sists primarily of calcium hydroxide (80%), along with sodium
hydroxide, water, and a small amount of potassium hydroxide. Its reactions are
as follows:
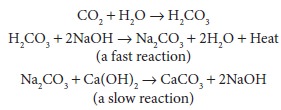
Note that the water and sodium hydroxide ini-tially required are regenerated. Another absorbent, barium hydroxide lime, is no longer used due to the possible increased hazard of fire in the breathing system.
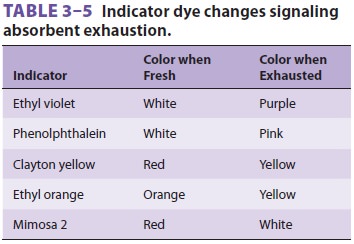
A
pH indicator dye (eg, ethyl violet) changes color from white to purple as a
consequence of increasing hydrogen ion concentration and absorbent exhaus-tion
(Table 3–5).
Absorbent should be replaced when 50% to 70% has changed color. Although
exhausted granules may revert to their original color if rested, no significant
recovery of absorptive capac-ity occurs. Granule size is a compromise between
the higher absorptive surface area of small granules and the lower resistance
to gas flow of larger granules. The granules commonly used as CO2
absorbent are between 4 and 8 mesh; the number of mesh corre-sponds to the
number of holes per square inch of a screen. The hydroxide salts are irritating
to the skin and mucous membranes. Increasing the hardness of soda lime by
adding silica minimizes the risk of inha-lation of sodium hydroxide dust and
also decreases resistance of gas flow. Additional water is added to absorbent
during packaging to provide optimal con-ditions for carbonic acid formation.
Commercial soda lime has a water content of 14% to 19%.
Absorbent
granules can absorb and later release medically important amounts of volatile
anesthetic. This property can be responsible for modest delays of induction or
emergence. The drier the soda lime, the more likely it will absorb and
degradevolatile anesthetics. Volatile anesthetics can be bro-ken down to carbon
monoxide by dry absorbent (eg, sodium or potassium hydroxide) to such a degree
that it is capable of causing clinically significant car-bon monoxide poisoning.
The formation of carbon monoxide is highest with desflurane; with sevoflu-rane,
it occurs at a higher temperature.Amsorb is a CO2 absorbent
consisting of cal-cium hydroxide and calcium chloride (with calcium sulfate and
polyvinylpyrrolidone added to increase hardness). It possesses greater
inertness than soda lime, resulting in less degradation of volatile
anes-thetics (eg, sevoflurane into compound A or desflu-rane into carbon
monoxide).
Compound
A is one of the by-products of deg-radation of sevoflurane by absorbent. Higher
con-centrations of sevoflurane, prolonged exposure, and low-flow anesthetic
technique seem to increase the formation of Compound A. Compound A has been
shown to produce nephrotoxicity in animals,
The
granules of absorbent are contained within one or two canisters that fit snugly
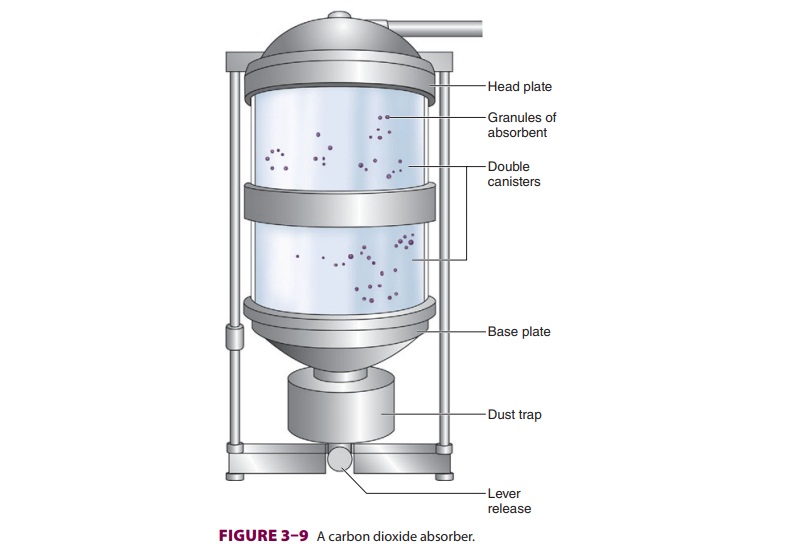
between a head and base plate. Together, this unit is called an absorber (Figure 3–9).
Although bulky, double
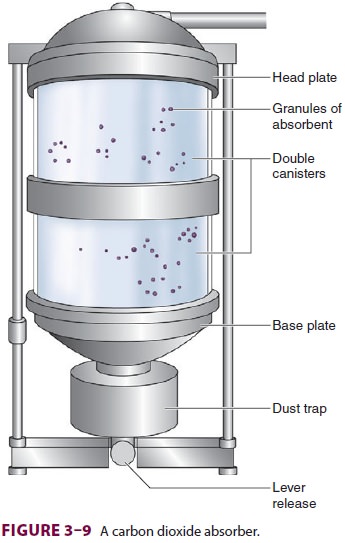
canisters
permit more complete CO2 absorption, less frequent absorbent
changes, and lower gas flow resistance. To ensure complete absorption, a
patient’s tidal volume should not exceed the air space between absorbent
granules, which is roughly equal to 50% of the absorber’s capacity. Indicator
dye color is monitored through the absorber’s trans-parent walls. Absorbent
exhaustion typically occurs first where exhaled gas enters the absorber and
along the canister’s smooth inner walls. Channeling through areas of loosely
packed granules is mini-mized by a baffle system, which directs gas flow
through the center, thereby allowing greater utili-zation of the absorbent. A
trap at the base of the absorber collects dust and moisture. Newer absorb-ers
are used until CO 2 is found in the inhaled gas on the
anesthetic-gas monitor, at which time the canister(s) are replaced.
B. Unidirectional Valves
Unidirectional
valves, which function as check valves, contain a ceramic or mica disk resting
hori-zontally on an annular valve seat ( Figure 3–10). Forward flow displaces the disk
upward, permitting the gas to proceed through the circuit. Reverse flow pushes
the disk against its seat, preventing reflux. Valve incompetence is usually due
to a warped disk or seat irregularities. The expiratory valve is exposed to the
humidity of alveolar gas. Condensation and resultant moisture formation may
prevent upward
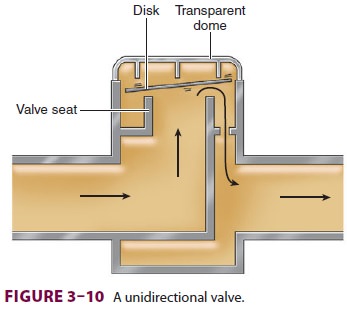
displacement
of the disks, resulting in incomplete escape of expired gases and
rebreathing.Inhalation opens the inspiratory valve, allowing the patient to
breathe a mixture of fresh and exhaled gas that has passed through the CO2
absorber. Simultaneously, the expiratory valve closes to pre-vent rebreathing
of exhaled gas that still contains CO2. The subsequent flow of gas
away from the patient during exhalation opens the expiratory valve. This gas is
vented through the APL valve or rebreathed by the patient after passing through
the absorber. Closure of the inspiratory valve during exhalation prevents
expiratory gas from mixing with fresh gas in the inspiratory limb. Malfunction
of either unidirectional valve may allowrebreathing of CO2,
resulting in hypercapnia.
Related Topics