Chapter: Basic & Clinical Pharmacology : Immunopharmacology
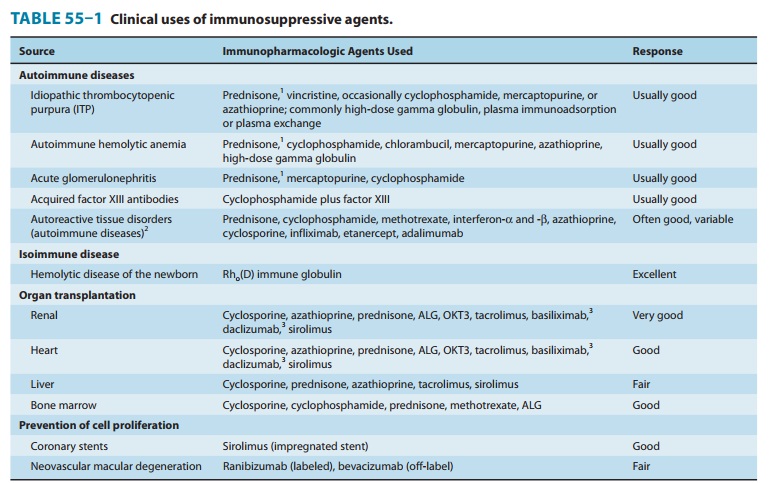
Clinical Uses of Immunosuppressive Drugs
CLINICAL USES OF IMMUNOSUPPRESSIVE DRUGS
Immunosuppressive
agents are commonly used in two clinical cir-cumstances: transplantation and
autoimmune disorders. The agents used differ somewhat for the specific
disorders treated (see specific agents and Table 55–1), as do administration
schedules. Because autoimmune disorders are very complex, optimal treatment
sched-ules have yet to be established in many clinical situations.
SOLID ORGAN & BONE MARROW TRANSPLANTATION
In organ
transplantation, tissue typing—based on donor and recipient histocompatibility
matching with the human leukocyte antigen (HLA) haplotype system—is required.
Close histocompat-ibility matching reduces the likelihood of graft rejection
and may also reduce the requirements for intensive immunosuppressive therapy.
Prior to transplant, patients may receive an immunosup-pressive regimen,
including antithymocyte globulin, muromonab-CD3, daclizumab, or basiliximab.
Four types of rejection can occur in a solid organ transplant recipient: hyperacute, accelerated,acute, and chronic. Hyperacute rejection is due
to preformedantibodies against the donor organ, such as anti-blood group
anti-bodies. Hyperacute rejection occurs within hours of the transplant and
cannot be stopped with immunosuppressive drugs. It results in rapid necrosis
and failure of the transplanted organ. Accelerated rejection is mediated by
both antibodies and T cells, and it also cannot be stopped by immunosuppressive
drugs. Acute rejection of an organ occurs within days to months and involves
mainly cellular immunity. Reversal of acute rejection is usually possible with
general immunosuppressive drugs such as azathioprine, myco-phenolate mofetil,
cyclosporine, tacrolimus, glucocorticoids, cyclo-phosphamide, methotrexate, and
sirolimus. Recently, biologic agents such as anti-CD3 monoclonal antibodies
have been used to stem acute rejection. Chronic rejection usually occurs months
or even years after transplantation. It is characterized by thickening and
fibrosis of the vasculature of the transplanted organ, involving both cellular
and humoral immunity. Chronic rejection is treated with the same drugs as those
used for acute rejection.
Allogeneic
hematopoietic stem cell transplantation is a well-established treatment for
many malignant and nonmalignant dis-eases. An HLA-matched donor, usually a
family member, is located, patients are conditioned with high-dose chemotherapy
or radiation therapy, and then donor stem cells are infused. The con-ditioning
regimen is used not only to kill cancer cells in the case of malignant disease,
but also to totally suppress the immune system so that the patient does not
reject the donor stem cells. As patients’ blood counts recover (after reduction
by the conditioning regimen) they develop a new immune system that is created
from the donor stem cells. Rejection of donor stem cells is uncommon, and can
only be treated by infusion of more stem cells from the donor.
Graft-versus-host
disease, however, is very common, occurring in the majority of patients who
receive an allogeneic transplant. Graft-versus-host disease occurs as donor T
cells fail to recognize the patient’s skin, liver, and gut (usually) as self
and attack those tissues. Although patients are given immunosuppressive therapy
(cyclosporine, methotrexate, and others) early in the transplant course to help
prevent this development, it usually occurs despite these medications. Acute
graft-versus-host disease occurs within the first 100 days, and is usually
manifested as a skin rash, severe diar-rhea, or hepatotoxicity. Additional
medications are added, invari-ably starting with high-dose corticosteroids, and
adding drugs such as mycophenolate mofetil, sirolimus, tacrolimus, daclizumab,
and others, with variable success rates. Patients generally progress to chronic
graft-versus-host disease (after 100 days) and require ther-apy for variable
periods thereafter. Unlike solid organ transplant patients, however, most stem
cell transplant patients are able to eventually discontinue immunosuppressive
drugs as graft-versus-host disease resolves (usually 1–2 years after their
transplant).
AUTOIMMUNE DISORDERS
The effectiveness of
immunosuppressive drugs in autoimmune disorders varies widely. Nonetheless,
with immunosuppressive therapy, remissions can be obtained in many instances of
autoim-mune hemolytic anemia, idiopathic thrombocytopenic purpura, type 1
diabetes, Hashimoto’s thyroiditis, and temporal arteritis. Improvement is also
often seen in patients with systemic lupus erythematosus, acute
glomerulonephritis, acquired factor VIII inhibitors (antibodies), rheumatoid
arthritis, inflammatory myo-pathy, scleroderma, and certain other autoimmune
states.
Immunosuppressive
therapy is utilized in chronic severe asthma, where cyclosporine is often
effective and sirolimus is another alternative. Omalizumab (anti-IgE antibody)
has been approved for the treatment of severe asthma (see previous section).
Tacrolimus is currently under clinical investigation for the man-agement of
autoimmune chronic active hepatitis and of multiple sclerosis, where IFN-β has a
definitive role.
IMMUNOMODULATION THERAPY
The development of
agents that modulate the immune response rather than suppress it has become an
important area of pharma-cology. The rationale underlying this approach is that
such drugs may increase the immune responsiveness of patients who have either
selective or generalized immunodeficiency. The major poten-tial uses are in
immunodeficiency disorders, chronic infectious diseases, and cancer. The AIDS
epidemic has greatly increased interest in developing more effective
immunomodulating drugs.
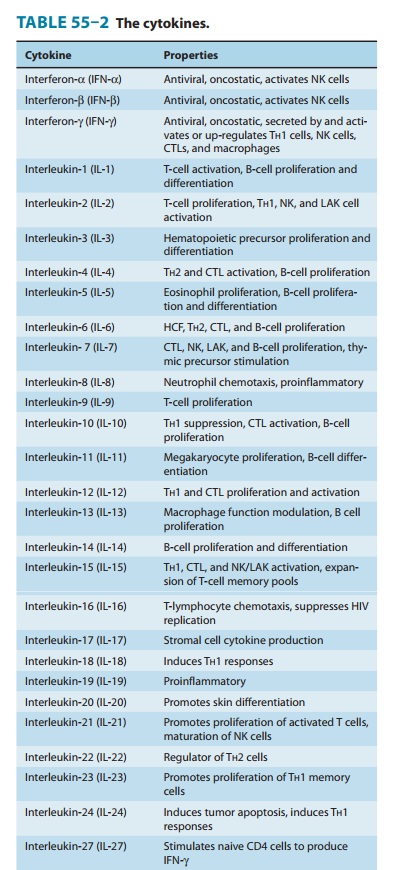
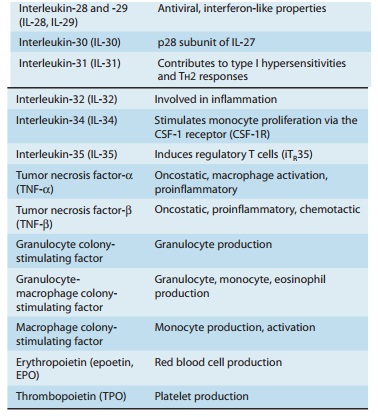
Cytokines
The cytokines are a
large and heterogeneous group of proteins with diverse functions. Some are
immunoregulatory proteins syn-thesized within lymphoreticular cells and play numerous
interact-ing roles in the function of the immune system and in the control of
hematopoiesis. The cytokines that have been clearly identified are summarized
in Table 55–2. In most instances, cytokines medi-ate their effects through
receptors on relevant target cells and appear to act in a manner similar to the
mechanism of action of hormones. In other instances, cytokines may have
antiprolifera-tive, antimicrobial, and antitumor effects.
The
first group of cytokines discovered, the interferons (IFNs), were followed by
the colony-stimulating factors. The latter regulate the proliferation and
differen-tiation of bone marrow progenitor cells. Most of the more recently
discovered cytokines have been classified as interleukins (ILs) and numbered in
the order of their discovery. Cytokines are produced using gene cloning
techniques.
Most
cytokines (including TNF-α, IFN-γ, IL-2, granulocyte
colony-stimulating factor [G-CSF], and granulocyte-macrophage
colony-stimulating factor [GM-CSF]) have very short serum half-lives (minutes).
The usual subcutaneous route of administration provides slower release into the
circulation and a longer duration of action. Each cytokine has its own unique
toxicity, but some toxicities are shared. For example, IFN-α, IFN-β, IFN-γ, IL-2, and
TNF-α
all induce fever, flu-like symptoms, anorexia, fatigue, and malaise.
Interferons
are proteins that are currently grouped into three families: IFN-`,
IFN-a,
and IFN-f.
The IFN-α
and IFN-β
fami-lies comprise type I IFNs, ie, acid-stable proteins that act on the same
receptor on target cells. IFN-γ, a type II IFN, is acid-labile and
acts on a separate receptor on target cells. Type I IFNs are usually induced by
virus infections, with leukocytes producing IFN-α. Fibroblasts and epithelial cells
produce IFN-β.
IFN-γ
is usually the product of activated T lymphocytes.
IFNs
interact with cell receptors to produce a wide variety of effects that depend
on the cell and IFN types. IFNs, particularly IFN-γ, display immune-enhancing
properties, which include increased antigen presentation and macrophage, NK
cell, and cytotoxic T-lymphocyte activation. IFNs also inhibit cell
prolifera-tion. In this respect, IFN-α and IFN-β are more potent than IFN-γ. Another
striking IFN action is increased expression of MHC molecules on cell surfaces.
While all three types of IFN induce MHC class I molecules, only IFN-γ induces
class II expres-sion. In glial cells, IFN-β antagonizes this effect and may,
in fact, decrease antigen presentation within the nervous system.
IFN-α is
approved for the treatment of several neoplasms, including hairy cell leukemia,
chronic myelogenous leukemia,malignant melanoma, and Kaposi’s sarcoma, and for
use in hepati-tis B and C infections. It has also shown activity as an
anticancer agent in renal cell carcinoma, carcinoid syndrome, and T-cell
leu-kemia. IFN-β
is approved for use in relapsing-type multiple sclero-sis. IFN-γ is
approved for the treatment of chronic granulomatous disease and IL-2, for
metastatic renal cell carcinoma and malignant melanoma. Clinical investigations
of other cytokines, including IL-1, -3, -4, -6, -10, -11, and -12, are ongoing.
Toxicities of IFNs, which include fever, chills, malaise, myalgias,
myelosuppression, headache, and depression, can severely restrict their
clinical use.
TNF-α has been extensively tested in the therapy of various malignancies, but results have been disappointing due to dose-limiting toxicities. One exception is the use of intra-arterial high-dose TNF-α for malignant melanoma and soft tissue sarcoma of the extremities. In these settings, response rates greater than 80% have been noted.
Cytokines
have been under clinical investigation as adjuvants to vaccines, and IFNs and
IL-2 have shown some positive effects in the response of human subjects to
hepatitis B vaccine. Denileukin diftitox is IL-2 fused to diphtheria toxin,
used for the treatment of patients with CD25+ cutaneous T-cell lymphomas. IL-12
and GM-CSF have also shown adjuvant effects with vac-cines. GM-CSF is of
particular interest because it promotes recruitment of professional
antigen-presenting cells such as the dendritic cells required for priming naive
antigen-specific T-lymphocyte responses. There are some claims that GM-CSF can
itself stimulate an antitumor immune response, resulting in tumor regression in
melanoma and prostate cancer.
It
is important to emphasize that cytokine interactions with target cells often
result in the release of a cascade of different endogenous cytokines, which
exert their effects sequentially or simultaneously. For example, IFN-γ exposure
increases the num-ber of cell-surface receptors on target cells for TNF-α. Therapy
with IL-2 induces the production of TNF-α, while therapy with IL-12 induces
the production of IFN-γ.
Cytokine Inhibitors
A
more recent application of immunomodulation therapy involves the use of
cytokine inhibitors for inflammatory diseases and septic shock, conditions in
which cytokines such as IL-1 and TNF-α (see above) are involved in the
pathogenesis. Drugs now in use or under investigation include anticytokine
antibodies and soluble cytokine receptors. Anakinra
is a recombinant form of the naturally occur-ring IL-1 receptor antagonist that
prevents IL-1 from binding to its receptor, stemming the cascade of cytokines
that would otherwise be released. Anakinra is approved for use in adult
rheumatoid arthri-tis patients who have failed treatment with one or more
disease-modifying antirheumatic drugs. Canakinumab
is a recombinant human anti-IL-1β monoclonal antibody. It binds to
human IL-1β
and prevents it from binding to IL-1 receptors. Rilonacept is a dimeric fusion protein consisting of the
ligand-binding domains of the extracellular portions of the human interleukin-1
receptor com-ponent (IL-1RI) and IL-1 receptor accessory protein (IL-1RAcP)
fused to the Fc portion of human IgG1. These molecules are indi-cated for
treatment of cryopyrin-associated periodic syndromes.
Patients
must be carefully monitored for serious infections or malignancies if they are
also taking an anti-TNF-α drug, have chronic infections, or
are otherwise immunosuppressed.
Related Topics