Chapter: Basic Concept of Biotechnology : Macromolecules and Analytical Techniques
Chromatography: Types, Principle, Application - Analysis of biomolecules
Chromatography
Chromatography serves as a means of separation of mixtures and for the isolation and partial description of the components whose presence may be known or to be identified. Chromatography was first employed by the Russian scientist Mikhail Tsvet in 1900. He separated chlorophyll II in 1906 by using thin layer chromatography. Like that of centrifugation Chromatography is also of preparative and analytical type. Preparative chromatography is used to separate the components of a mixture for further studies or for purification. Whereas, analytical chromatography is done normally with smaller amounts of material and is for measuring the relative proportions of analytes in a mixture. The concept of partition coefficient is the basic principle of chromatography. In chromatography, the components to be separated are distributed between a stationary phase and themobile phase in which a stationary phase could be paper (paper chromatography), silica (thin layer and liquid chromatography), liquid silicone (gas liquid chromatography) etc and the mobile phase could be polar and non polar solvents and inert gases etc. Separation of the components in a sample is based on the fact that the rate of travel of an individual solute molecule through a column or thin layer of adsorbent is directly related to the partition of that molecule between the mobile and stationary phase. The partition coefficient of each component determines how much of it is in each phase at any time and how long it remains in that phase. After some time interval they will be distributed in space over the stationary phase and subsequently emerge out of the stationary phase as a single components.
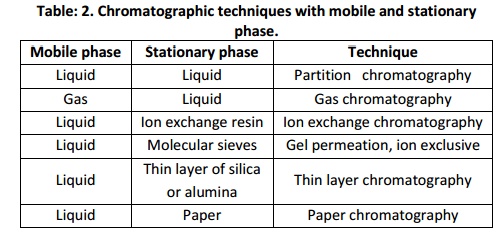
The choice of chromatographic technique solely depend on the purpose of the experiment in which the solute particle or the analytes are to be isolated, separated, estimated, to be identified, for characterization and structural elucidation etc. Hence chromate graphic techniques of various types viz; Column chromate graphy, Gas chromatography, High performance liquid chromatography, Affinity chromatography, Ion exchange chromatography, Thin layer chromatography, Gel permeation chromatography and etc does exist.
Since High performance Liquid chromatography and Gas chromatography are extensively used in research and chemical industries the brief description of them is mentioned in the following session.
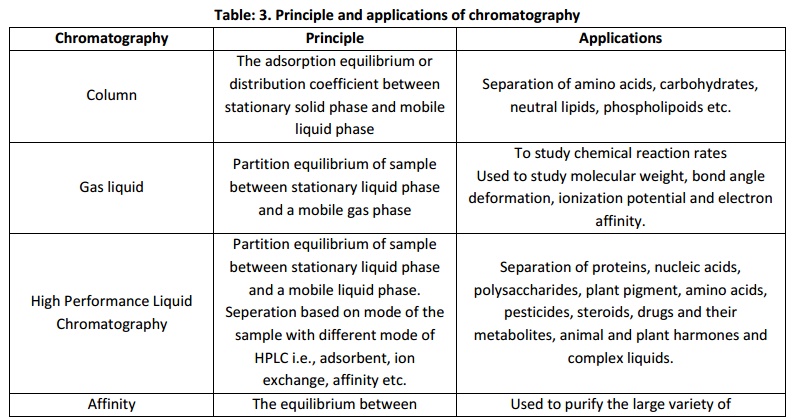
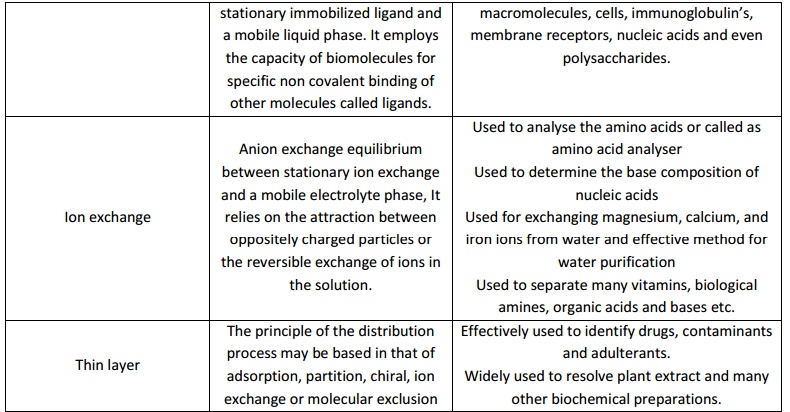
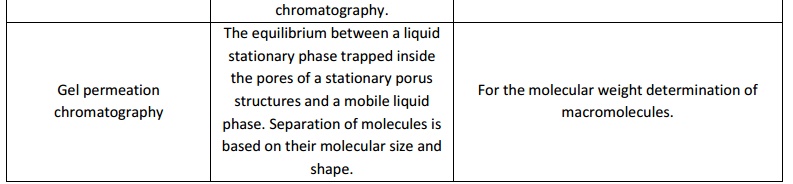
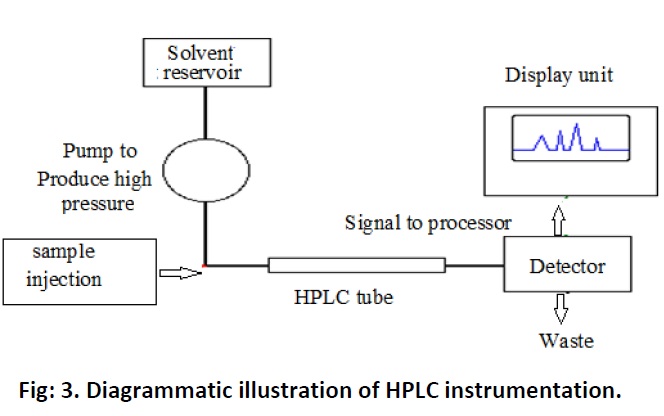
High pressure liquid chromatography (HPLC)
Generally the instrumentation part consists of high pressure pumps which can pump the mobile phase at 0.01 to 10ml/min at the pressure up to 500bars. HPLC contain 6 port injection valve from which sample mixture is injected. The samples should be miscible with the mobile phase. However before injecting the samples they should be filtered and processed. The mobile phase reservoirs are placed on top of the instrument. In normal phase HPLC a mobile phase is non polar e.g., hydrocarbons whereas a stationary phase is polar for e.g., AL2O3, SI02. In case of reverse phase HPLC mobile phase is polar such as water, acetonitrile or mixture of them and etc, and the stationary phase is non polar. The run time is usually 5 to 60 min depending on the sample. After run is completed the samples enter to the detectors. There are different types of detectors are available based on requirement of studies. UV visible or Diode array detector is used to study the larger organic molecules and transition metals which absorb the UV visible light. Fluorescence detectors are used for highly condensed organic molecules and it detects fluorescence radiation emitted by the sample compounds. Other types of detectors are Refractive index detector which is low cost and non specific, electric conductivity detector for compound dissociated into ions and Mass Selective detector.
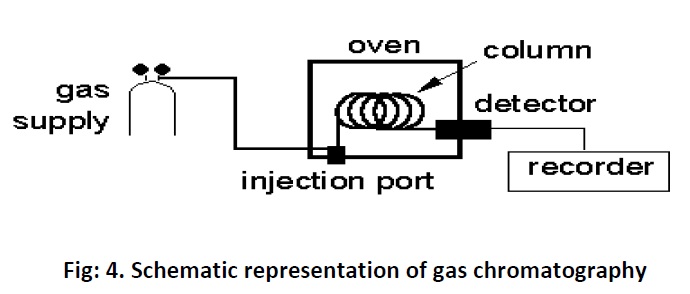
Gas chromatography (GC)
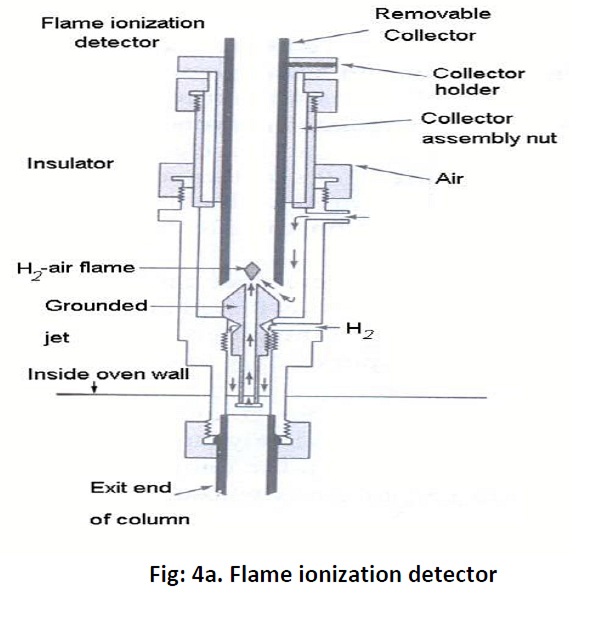
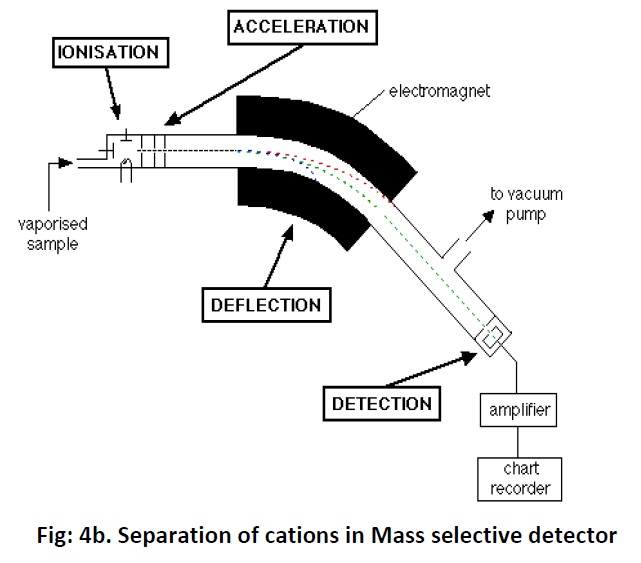
Gas chromatography consists of flow controllers which control the flow of the mobile phase which is an inert gas such as Helium, nitrogen, argon and etc of purity 99.999%. The instrument has an injection port through which samples are injected. The samples may be in gas form or in liquid form.Theinjection port is maintained at a higher temperature than the boiling point of the least volatilecomponent in the sample mixture. However the sample which has to be analysed should be stable under GC operation conditions and should have a vapour pressure higher than zero. In standard GC the analysis or run time may be between 5 to 60 min whereas it is less than 2 min in micro GC. Once the sample injected they will be vaporized and pushed through the column and get separated. Here the columns are of two types viz; capillary and packed columns. Capillary columns diameter ranges from 0.1 to 0.53 mm and length from 10 to 50 meters and are made up of thin fused silica. Inside the column a thin layer of stationary phase is coated measuring about 0.3 to 50 micrometer thickness. The stationary phase could be silicon polymers (polysiloxanes Si-O-R) where R could be methyl, phenyl, cyanopropyl, ethylene glycol or fluorinated hydrocarbon. The packed columns are having a diameter of 0.53 mmand length of 1 to 5 meters; typically they are made up of a glass or stainless steel coil. The small particles inside the column acts as stationary phase and the particle diameter would be 45-120 mesh. These small particles are immobilized in the wall for separation of high volatile compounds. Some of the stationary phase is AL2O3, polystyrene divinylbenzene (DVB). Once the samples get separated they will enter into the detectors. The important detectors of GC are Thermal Conductivity Detector (TCD) which is a non selective one and which can detect anything that differs from the carrier gas. Flame ionization detector (FID) Fig: 4a is far the most widely used detector. It measures all organic compounds and it can detect as low as one nanogram of any given compounds. It consists of a hydrogen/air flame and a collector plate.The efflucent from the GC column passes through the flame, which breaks down organic molecule and produces ions. The ions are collected on a biased electrode and produce an electric signal. It is extremely sensitive and has large dynamic range.Another type of detector is Electron capture detector (ECD) which has a radioactive source which ionizes the carrier gas coming out of the column. When an organic molecule that contains electornegative functional group, such as halogens, phosphorous and nitro groups, pass by the detector they capture some of the electrons and reduce the current. It is mostly used to measure polyhalogenated compounds particularly pesticides. It is very sensitive and can detect as little as one picogram of these compounds. The important and advance detector is Mass Selective Detector (MSD) Fig: 4b which is tunable for any species of sample or ions. It uses the difference in mass-to-charge ratio (m/z) of ionized atoms or molecules to separate them from each other.Itcreate gas-phase ions and separate the ions in space or time based on their mass to charge ratio then measure the quantity of ions.
Applications
Identification and quantitation of volatile and semivolatile organic compounds in complex mixtures.Determination of molecular weights and (sometimes) elemental compositions of unknown organic compounds in complex mixtures.Structural determination of unknown organic compounds in complex mixtures both by matching their spectra with reference spectra and by a priori spectral interpretation.
Related Topics