Chapter: Medicine and surgery: Respiratory system
Bronchial carcinoma - Respiratory oncology
Respiratory oncology
Bronchial carcinoma
Definition
A malignant tumour of the bronchial (most common) or rarely alveolar epithelium.
Incidence
Bronchial carcinoma is the most common malignancy of the Western World: 40,000 deaths per year in the United Kingdom, more than 1 million deaths worldwide.
It is the leading cause of death from cancer in men and in women under the age of 65, it has now exceeded breast cancer as the leading cause of death from cancer.
Age
Peak age 40–70 years.
Sex
3M : 1F, but rising in females.
Geography
Follows patterns of smoking, independent of this it is also higher in urban areas.
Aetiology
Around 80–90% of cases occur in smokers (see Table 3.21).
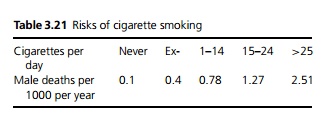
It takes an exsmoker of ≤20 per day 13 years to return to just above the risk of a non-smoker. Pipe smokers have about 40% the risk of cigarette smokers.
Occupational and environmental factors include exposure to radioactive material (including radon), asbestos, nickel, chromium, iron oxides and coal gas plants.
Pathophysiology
Lung cancer is characterised by multiple genetic alterations:
i. In >90% of small cell lung cancers the p53 and rb tumour suppressor genes are both mutated, and >50% and 20% respectively in non-small cell lung cancer.
ii. Activation of dominant oncogenes – for example, mutations in ras genes are associated with 20% of non-small cell lung cancer and confer a poor prognosis, while tumour amplification of c-myc is associated with a poor prognosis in small cell lung cancer.
iii. Some of these genetic alterations are seen in pre-neoplastic lesions such as hyperplasia, dysplasia and carcinomainsitu of the bronchial epithelium, but it appears that as many as 10 of these mutations are needed for the development of lung cancer.
Clinical features
Cough or worsening of a pre-existing cough is the most common early symptom, but may be ignored. Haemoptysis due to ulceration of a tumour is the next most common. Dyspnoea, a dull central chest ache or pleuritic pain, or slowly resolving chest infection are all common. Clubbing and systemic features (weight loss, anorexia and malaise) or complications from metastatic deposits may also be presenting features.
Macroscopy/microscopy
Because of their pathological behaviour malignancies of the lung are divided into ‘small cell’ and ‘non-small cell’. This is useful clinically on deciding treatment. But histologically, lung carcinoma is divided into four cell types:
i. Squamous cell carcinoma, 50%.
ii. Small/oat cell carcinoma, 20%.
iii. Adenocarcinoma, 20%.
iv. Large cell anaplastic carcinoma, 10%.
A few show a mixed pattern: 70% of all tumours arise in relation to the main bronchus (central or hilar) and 30% arise in the peripheral airways or alveoli.
1. 1 Squamous cell carcinoma: Usually located centrally close to the carina and so presents with the sequelae of bronchial obstruction and local invasion. Lesions may cavitate. Histologically squamous cell carcinoma shows a variety of patterns from well-differentiated lesions producing lots of keratin to poorly differentiated lesions containing little keratin.
2. Small cell/oat cell/anaplastic lung cancer is a highly malignant tumour arising from bronchial epithelium, but with properties of neuroendocrine cells containing secretory granules. Tumours are centrally located and are associated with a rapid growth rate with metastases almost invariable at presentation. Often associated with ectopic ADH and ACTH secretion (water/sodium retention and Cushing’s syndrome).
3. Adenocarcinoma characteristically develops as a peripheral tumour, but may arise from the main bronchus. A proportion are thought to arise from pre-existing lung scars. It is the most common bronchial carcinoma associated with asbestos and is proportionally more common in non-smokers. Histologically four patterns are seen:
- Acinar – prominent gland-like spaces.
- Papillary – fronds of tumour on thin septa.
- Solid carcinoma – poorly differentiated with mucin production.
- Alveolar cell carcinoma derived from alveolar or bronchial epithelial cells (Clara cells and type II pneumocytes). These may exist as isolated peripheral nodules, but they characteristically spread through the lung along alveolar septa (seen on chest X-ray as areas of alveolar shadowing). Half are multifocal infiltrative tumours, which replace areas of lung in a manner resembling pneumonic consolidation. Cells are tall, columnar and relatively uniform, have few mitoses and secrete mucin (sometimes copious). The remaining half are single grey masses up to 10 cm in diameter made up of cuboidal cells with hyperchromatic nuclei.
1. Large cell anaplastic carcinomas are poorly differentiated lesions composed of large cells with nuclear pleomorphism and frequent giant cell forms.
Complications
1.Intra-thoracic: Distal pneumonia, lobar collapse and consolidation, pleural effusions, left recurrent laryngeal nerve palsy (hoarse voice), superior vena cava obstruction, brachial neuritis (particularly apical tumour (Pancoast tumour)), Horner’s syndrome (sympathetic paralysis causing partial ptosis, myosis, anhydrosis, enophthalmos), rib erosion, pericarditis, oesophageal obstruction.
2.Metastases: Haematogenous spread to bone (pain or fractures), brain, liver, adrenal gland.
3.Endocrine (10%, usually small cell carcinoma): Antidiuretic hormone, ectopic ACTH secretion. Hyper-calcaemia seen with squamous cell carcinoma is due to secretion of a parathyroid hormone related peptide (PTH-like peptide).
4.Neuromuscular: Neuropathy, myopathy, myositis, dementia, cerebellar degeneration.
5.Eaton Lambert syndrome: Rare non-metastatic manifestation of small cell carcinoma causing defective acetylcholine release at the neuromuscular junction resulting in proximal muscle weakness with absent reflexes.
6. Systemic: Weight loss, anaemia, clubbing, hyper-trophic pulmonary osteoarthropathy (HPOA – this is clubbing associated with peripheral joint pain and stiffness in the wrists and ankles caused by a periostitis. It tends to occur more often in squamous cell and adenocarcinoma).
Investigations
- X-ray evidence only when 1–2 cm (still identifies over 90% of carcinomas). The edge of the lesion appears typically fluffy or spiked, some may cause cavitation or collapse. Hilar node enlargement or effusions are sometimes evident.
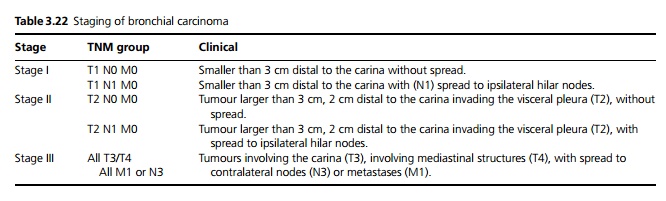
- CT is useful for small lesions but not yet proven to be useful in widespread screening. It is mainly used to assess the extent and spread, especially lymph nodes (see Table 3.22).
- Sputum cytology: Examination of expectorated sputum by cytology.
- Cytology of pleural effusion: Examination of pleural fluid.
- Percutaneous needle aspiration/biopsy under CT guidance. Open lung biopsy may be needed, particularly for alveolar cell carcinoma.
- Bronchoscopy and biopsy.
Management
1. Identification of histological type is essential.
2. Surgery for all non-small cell carcinomas where possible. Surgical resection may be attempted in limited alveolar cell carcinoma.
3. Chemotherapy and adjuvant radiotherapy is considered in all patients, although chemotherapy is less effective in non-small cell carcinoma.
4. Palliative radiotherapy, laser therapy and tracheobronchial stents.
Surgical treatment: Key elements that offer a reasonable prospect of success are tumour within a lobar bronchus or at least 2 cm distal to the carina.
- no involvement of the heart, great vessels, trachea, oesophagus or vertebrae.
- no malignant pleural effusion.
- no contralateral node involvement.
- no distant metastases (chest CT, serum alkaline phosphatase).
- adequate lung function (FEV1 > 1.5 L, gas transfer >50% normal).
Surgery (only usually possible in 25%) involves the removal of the anatomical unit containing the tumour (segment, lobe or lung) together with the associated lymphatic drainage. 35–40% 5-year survival rate but closer to 90% with peripheral lesions.
Prognosis
· Non-small cell – 20% have successful resection, 10% survive for 5 years. Inoperable non-small cell carcinoma – chemotherapy is effective in ∼20% and at best gives a survival benefit of a few months, and it has toxic side-effects. Median survival ∼8 months with combination chemotherapy.
· Small cell carcinoma limited to thorax: Median survival 18–24 months with combination chemotherapy and radiotherapy and up to 20% survive 2 years, only 6–12 weeks without treatment. Small cell carcinoma with metastases: Median survival ∼8 months with combination chemotherapy, rarely survive to 2 years.
· The rare alveolar carcinoma carries a poor prognosis due to its multifocal nature.
Related Topics