Chapter: Basic & Clinical Pharmacology : The Alcohols
Basic Pharmacology of Ethanol: Pharmacokinetics
BASIC PHARMACOLOGY OF ETHANOL
Pharmacokinetics
Ethanol
is a small water-soluble molecule that is absorbed rapidly from the
gastrointestinal tract. After ingestion of alcohol in the fasting state, peak
blood alcohol concentrations are reached within 30 minutes. The presence of
food in the stomach delays absorp-tion by slowing gastric emptying.
Distribution is rapid, with tissue levels approximating the concentration in
blood. The volume of distribution for ethanol approximates total body water
(0.5–0.7 L/kg). For an equivalent oral dose of alcohol, women have a higher
peak concentration than men, in part because women have a lower total body
water content and in part because of differences in first-pass metabolism. In the
central nervous system (CNS), the concentra-tion of ethanol rises quickly,
since the brain receives a large pro-portion of total blood flow and ethanol
readily crosses biologic membranes.
Over
90% of alcohol consumed is oxidized in the liver; much of the remainder is
excreted through the lungs and in the urine. The excretion of a small but
consistent proportion of alcohol by the lungs can be quantified with breath
alcohol tests that serve as a basis for a legal definition of “driving under
the influence” in many countries. At levels of ethanol usually achieved in
blood, the rate of oxidation follows zero-order kinetics; that is, it is
indepen-dent of time and concentration of the drug. The typical adult can
metabolize 7–10 g (150–220 mmol) of alcohol per hour, the equivalent of
approximately one “drink” [10 oz (300 mL) beer, 3.5 oz (105 mL) wine, or 1 oz
(30 mL) distilled 80-proof spirits].
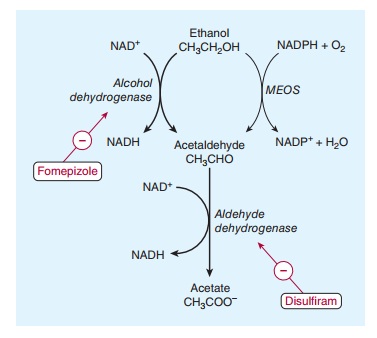
Two
major pathways of alcohol metabolism to acetaldehyde have been identified
(Figure 23–1). Acetaldehyde is then oxidized to acetate by a third metabolic
process.
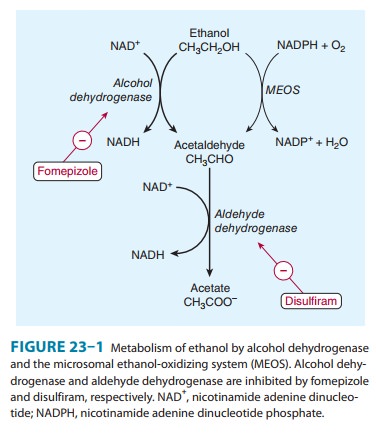
A. Alcohol Dehydrogenase Pathway
The
primary pathway for alcohol metabolism involves alcohol dehy-drogenase (ADH), a
family of cytosolic enzymes that catalyze the conversion of alcohol to
acetaldehyde (Figure 23–1, left). These enzymes are located mainly in the
liver, but small amounts are found in other organs such as the brain and
stomach. There is considerable genetic variation in ADH enzymes, affecting the
rate of ethanol metabolism and also appearing to alter vulnerability to
alcohol-abuse disorders. For example, one ADH allele (the ADH1B*2 allele), which is associated with rapid conversion of
ethanol to acet-aldehyde, has been found to be protective against alcohol
dependence in several ethnic populations and especially East Asians.
Some metabolism of ethanol by ADH occurs in the stomach in men, but a smaller amount occurs in women, who appear to have lower levels of the gastric enzyme. This difference in gastric metabolism of alcohol in women probably contributes to the sex-related differences in blood alcohol concentrations noted above.
During
conversion of ethanol by ADH to acetaldehyde, hydro-gen ion is transferred from
ethanol to the cofactor nicotinamide adenine dinucleotide (NAD+) to
form NADH. As a net result, alcohol oxidation generates an excess of reducing
equivalents in the liver, chiefly as NADH. The excess NADH production appears
to contribute to the metabolic disorders that accompany chronic alcoholism and
to both the lactic acidosis and hypoglyce-mia that frequently accompany acute
alcohol poisoning.
B. Microsomal Ethanol-Oxidizing System (MEOS)
This
enzyme system, also known as the mixed function oxidase system, uses NADPH as a
cofactor in the metabolism of ethanol (Figure 23–1, right) and consists
primarily of cytochrome P450 2E1, 1A2, and 3A4 .
During
chronic alcohol consumption, MEOS activity is induced. As a result, chronic
alcohol consumption results in sig-nificant increases not only in ethanol
metabolism but also in the clearance of other drugs eliminated by the
cytochrome P450s that constitute the MEOS system, and in the generation of the
toxic byproducts of cytochrome P450 reactions (toxins, free radicals, H2O2).
C. Acetaldehyde Metabolism
Much
of the acetaldehyde formed from alcohol is oxidized in the liver in a reaction
catalyzed by mitochondrial NAD-dependent aldehyde dehydrogenase (ALDH). The
product of this reaction is acetate (Figure 23–1), which can be further
metabolized to CO2 and water, or used to form acetyl-CoA Oxidation
of acetaldehyde is inhibited by disulfiram,
a drug that has been used to deter drinking by patients with alcohol
dependence. When ethanol is consumed in the presence of disul-firam,
acetaldehyde accumulates and causes an unpleasant reaction of facial flushing,
nausea, vomiting, dizziness, and headache. Several other drugs (eg,
metronidazole, cefotetan, trimethoprim) inhibit ALDH and can cause a
disulfiram-like reaction if com-bined with ethanol.
Some
people, primarily of East Asian descent, have genetic deficiency in the
activity of the mitochondrial form of ALDH, which is encoded by the ALDH2 gene. When these individuals drink
alcohol, they develop high blood acetaldehyde concentra-tions and experience a
noxious reaction similar to that seen with the combination of disulfiram and
ethanol. This form of ALDH, with reduced activity, is strongly protective
against alcohol-use disorders.
Related Topics